(C) 2013 Luiza Bertelli Simões. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The karstic area of São Domingos, central Brazil, holds extensive drainage systems. In order to understand its biodiversity, various volumes of water were filtered with planktonic nets in stretches of subterranean and superficial rivers on five different occasions. We sampled four drips (152L), three calcite pools (368L), two subterranean rivers fed mainly by percolation water (6, 395L), two subterranean rivers fed mainly by water coming from a sinkhole (4, 175L) along different caves, one resurgence (158L), and four epigean rivers (101, 690L). Physical and chemical variables were measured at some sites. Canonical Correlation Analysis was used to verify relationships between taxa and environment. The degree of similarity of the biota was assessed by cluster analysis (Sorensen, single linkage). There were records of exclusive taxa in epigean and subterranean samples, mainly in drips, which harbour the most unique fauna. The high richness of taxa presently recorded reveals the potential of the vadose zone biota in the tropical region, which was neglected in studies on Brazilian subterranean biodiversity. According to our results, the unsaturated zone tropical fauna may have different composition compared to that from temperate habitats. The studied communities were dominated by rotifers, while crustacean are predominant in the latter. The hypothesis can be clarified with the increase of long term studies and taxa identification at species level, besides the use of complementary sampling methods.
Subterranean habitats, epigean rivers, aquatic biota, Rotifera, conservation, Neotropical region
Most of the subterranean habitats develop in karst systems, which are discontinuous geomorphic systems formed in soluble rocks and characterized by the presence of aquifers and conduits, with subterranean drainage (
The waters that refuel the epikarst can infiltrate through different voids (i.e. fractures, conduits or shafts), which vary in physical and chemical properties (
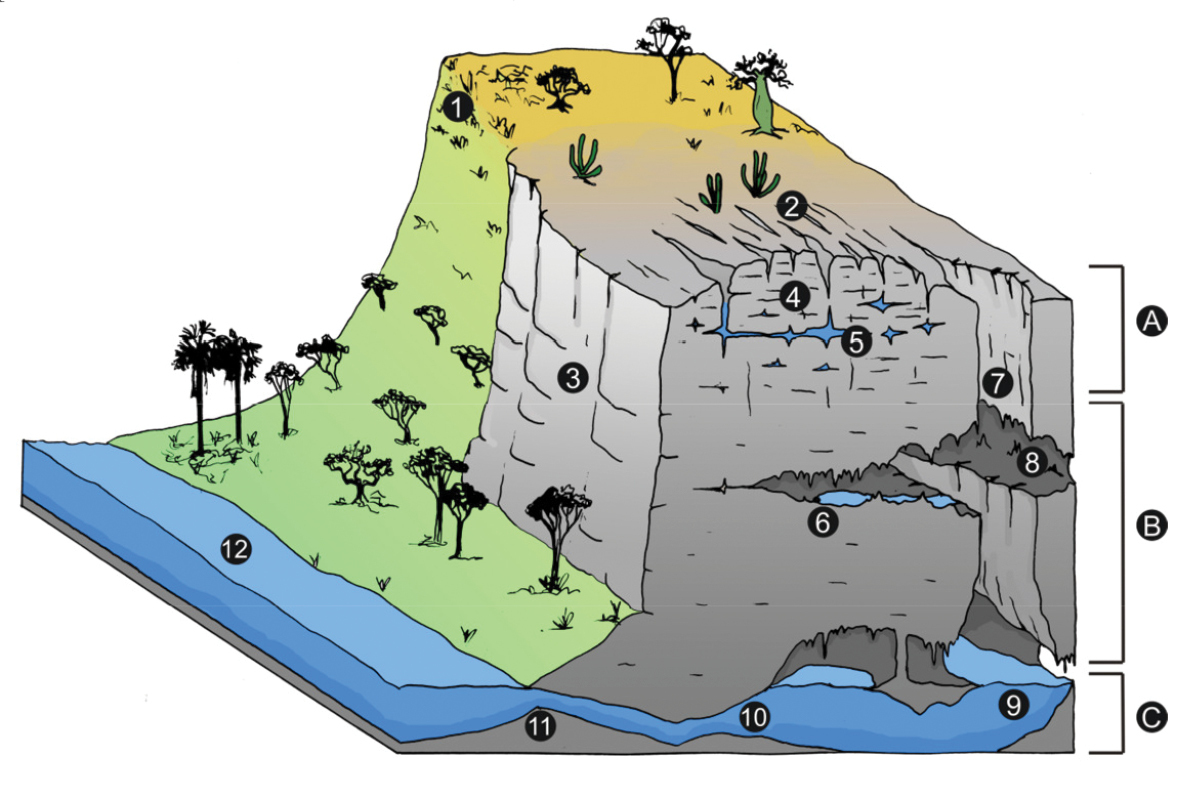
Horizons in karst: 1 soil 2 karst terrain 3 limestone outcrop; 4 epikarst 5 aquifer in epikarst 6 drips 7 doline 8 cave 9 and 10 subterranean river at the base level 11 resurgence 12 epigean river A epikarstic zone B vadose zone C phreatic or saturated zone. (Ilustration: Pedro Pereira Rizzato).
In these habitats an exceptionally rich fauna occurs, best described in some European karst areas (
The vadose zone fauna in temperate zones is mainly composed of small crustaceans, oligochaetes, nematodes, acari, and molluscs, less than 1 mm to several centimetres in body size (Gilbert et al. 2009). In general, stygobiotic biodiversity in Europe is dominated by Crustacea (
As the terrestrial hypogean fauna is more visible and can be easily assessed, it has been studied in more detail in Brazil (
The unique long-term study focusing on Brazilian subterranean aquatic fauna was conducted for fish populations (
Many studies have demonstrated that organic carbon may arrive in the form of animals dripping into caves through the epikarst (
As the study of the unsaturated zone biota is a new challenge for subterranean biology in Brazil, the main goal of this study was to elucidate part of the richness and distribution of the aquatic biota in different karstic horizons of São Domingos region and its surroundings, north-eastern Goiás state, central Brazil. Furthermore, we aimed to verify the correlation between this biota and some physical and chemical variables of the different habitats. The question of relevance versus visibility of this biota and its respective habitats was raised, considering that less visible species are yet important to the functioning of subterranean systems.
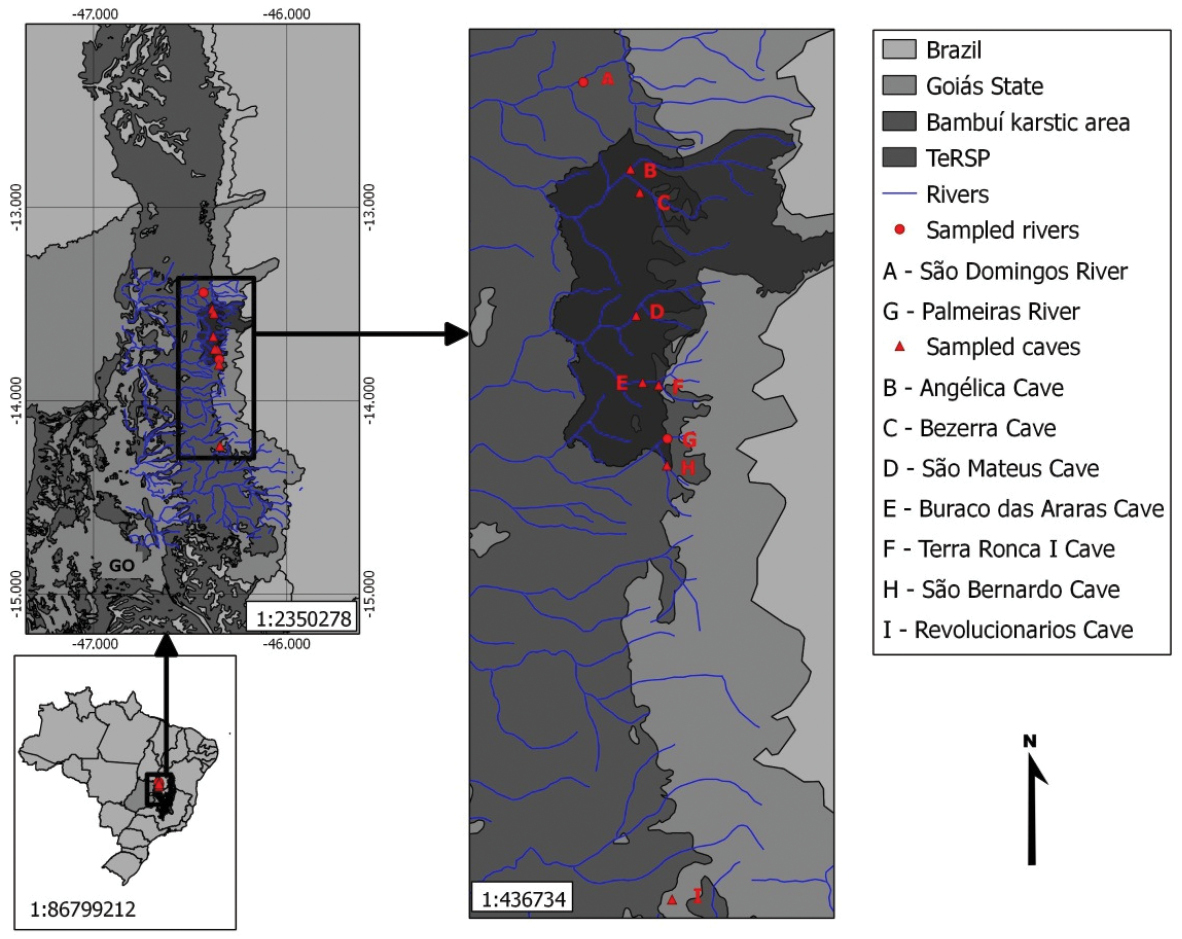
The karst area of São Domingos and its surroundings represents one of the regional formations of the Bambuí geomorphologic unit, which constitutes the largest set of limestone areas favourable to caves development in Brazil. The region has an approximate area of 105, 200 km2. In the region there are five large systems of caves, with up to 23 km of development (Figure 2).
Study area map with details of subterranean cave systems and epigean rivers at São Domingos karst area and surroundings, Goiás state, central Brazil. In dark gray – limits of Terra Ronca State Park (TeRSP). A São Domingos river B Angélica Cave C Bezerra Cave D São Mateus Cave E Buraco das Araras Cave F Terra Ronca I Cave (sinkhole of Lapa river) G Palmeiras river H São Bernardo Cave I Revolucionários Cave (this cave is located outside the limits of Terra Ronca State Park).
Superficial rivers belonging to the Paranã Basin (Alto Tocantins) penetrate the limestone layers after draining an extensive arenitic region, forming large cave systems (
The area is part of a state-level Conservation Unit (Terra Ronca State Park - TeRSP), created 17 years ago but still with diverse land ownership problems. One aggravating factor is that some river sources that cross the cave systems are located outside the park. There are anthropic activities as livestock, extensive plantations and clandestine mining which on long-term are responsible for the adverse effects on local drainage systems, which include the subterranean domain.
The studied region belongs to the morphoclimatic Dominion of the Cerrados (
The sampling area embodies subterranean systems and epigean rivers, sampled differently regarding frequency and volume since in some cases the availability of water was restricted. Abbreviations and characteristics of each sampled point are described in Table 1.
Characteristics, volumes and months in which sampling was undertaken. Sampling sites in the karst area of São Domingos and surroundings (2011-2012). Caves: Angélica (Ang), Buraco das Araras (BAra), Bezerra (Bez), São Bernardo (SBer), São Mateus (SMat), Revolucionários (Rev); epigean rivers: Palmeiras (Palm), Angélica (Ang), São Domingos (SD); resurgence: Terra Ronca (RTR). D = drip; Ek = subterranean river fed mainly by percolation water; Ep = epigean; P =pool; S = subterranean. *Locality visited on the five occasions (segment of Angélica Cave, monitoring base); samplings were not performed during periods of inactive drips.
| Points | CollectionOccasions | Vol (L) | Characteristics |
|---|---|---|---|
| AngD01 | Apr/2011; Feb/2012 * | 83 | Drips in the entrance zone of Angélica Cave. Water drips directly from the ceiling, with flow varying according to the season. |
| AngD02 | Apr/2011* | 3 | Reduced dripping, with low flow rate, and ceasing during the dry season. |
| AngEk | Apr & Oct/2011; Feb, Jun & Oct/2012 | 3, 596 | River fed by percolation water, habitat of the small catfish Ituglanis epikarsticus Bichuette & Trajano, 2004. The water flows with moderate rate and little turbulence, and there is notable reduction in the river level during the dry periods. |
| AngEp | Jun/2012 & Oct/212 | 23, 580 | Epigean Angélica River, point close to the sinkhole. |
| BAraD | Oct/2011 & Oct/12 | 21 | Water drips from ceiling speleothems. |
| BAraP | Oct/2011 & Oct/2012 | 45 | Pool near the BurAraGot drip. |
| BAraEp | Oct/2012 | 6, 000 | Epigean Lapa River, entrance of Buraco das Araras Cave. |
| BezD | Jun/2012 | 45 | Drips coming directly from the ceiling. |
| BezP | Jun/2012 | 113 | Pool in the aphotic zone of Bezerra Cave. Water drips from the ceiling and remains puddled. |
| BezS | Feb & Jun/2012 | 2, 603 | Subterranean river in the aphotic zone of Bezerra Cave, base level. |
| SBerEk | Fev/2012 | 2.592 | River in the aphotic zone of São Bernardo Cave. |
| SMatP | Feb/2012 | 210 | Pool in São Mateus Cave. Water drips from the ceiling and forms a puddle. |
| RTR | Oct/2011 | 158 | Lapa River at its epigean portion, resurgence of Terra Ronca Cave. |
| PalmEp | Apr/2011 | 110 | Palmeiras River (epigean) near the sinkhole of São Bernardo Cave. |
| SDEp | Feb & Jun/2012 | 72, 000 | São Domingos River (epigean), near its sinkhole. |
| RevS | Jun/2012 | 1, 545 | Subterranean river in Revolucionários Cave. |
The region was sampled on five occasions: April and October 2011, and February, June and October 2012. The subterranean stretches included drips, subterranean rivers (base level) and water pools (Figure 3). The sampled caves and rivers are: Angélica, Bezerra, Buraco das Araras, São Bernardo, São Mateus and Revolucionários caves; the epigean Palmeiras, Angélica, da Lapa, São Domingos Rivers, and the Resurgence of Terra Ronca (Table 1). Revolucionários Cave and São Domingos River are outside the park area, but inserted in the same limestone lens. The Angélica Cave is the only locality sampled on every trip, as a long-term monitoring base.
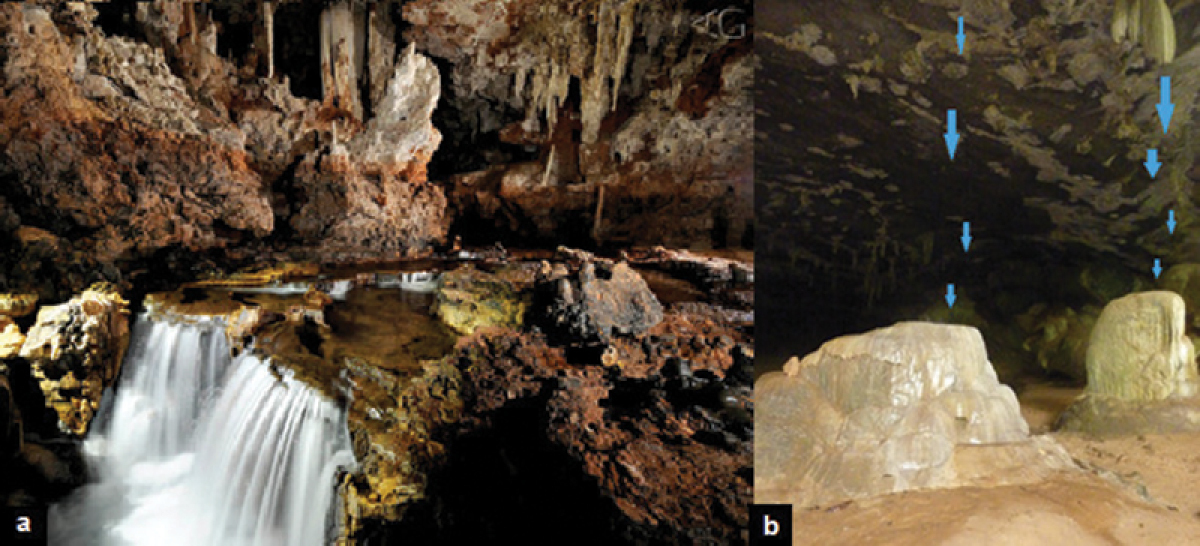
Figure 3. Subterranean river (A São Bernardo Cave) and drips (in blue) formed by infiltration water (B Angélica Cave – AngD01). Photography: a, Adriano Gambarini; b, Maria Elina Bichuette.
Samples were taken with plankton nets (20µm mesh), which remained installed for periods varying from twenty minutes to three hours, according to the local conditions. Nets, installed under dripping water sites based on the device proposed by
The following physical and chemical variables of water were measured by using a Horiba multiprobe (model U50G): temperature (°C), pH, ORP redox potential (mv), conductivity (mS/cm), dissolved oxygen (mg/L), oxygen saturation (%), total of dissolved solids TDS (g/L), turbidity (NTU), depth (m) and salinity (%).
Relative taxa richness among sites was assessed. Furthermore, the richness of the taxa in epigean and hypogean environments was taken into account separately for comparison. Similarity in composition of aquatic invertebrates in the different habitats was analysed by cluster analysis (Sorensen, single linkage).
In order to study the species-environment relationship, Canonical Correspondence Analysis (CCA) was used, associating the measured environment variables with the matrix of taxa present at eleven sampled sites.
Finally, by means of simple linear regression (Pearson correlation), we verified the relationship between the sampling effort and the richness of taxa, as the volumes sampled in each segment varied. Analyses of similarity and CCA were performed using the software PAST (version 2.13) and R language (version 3.0.1), respectively.
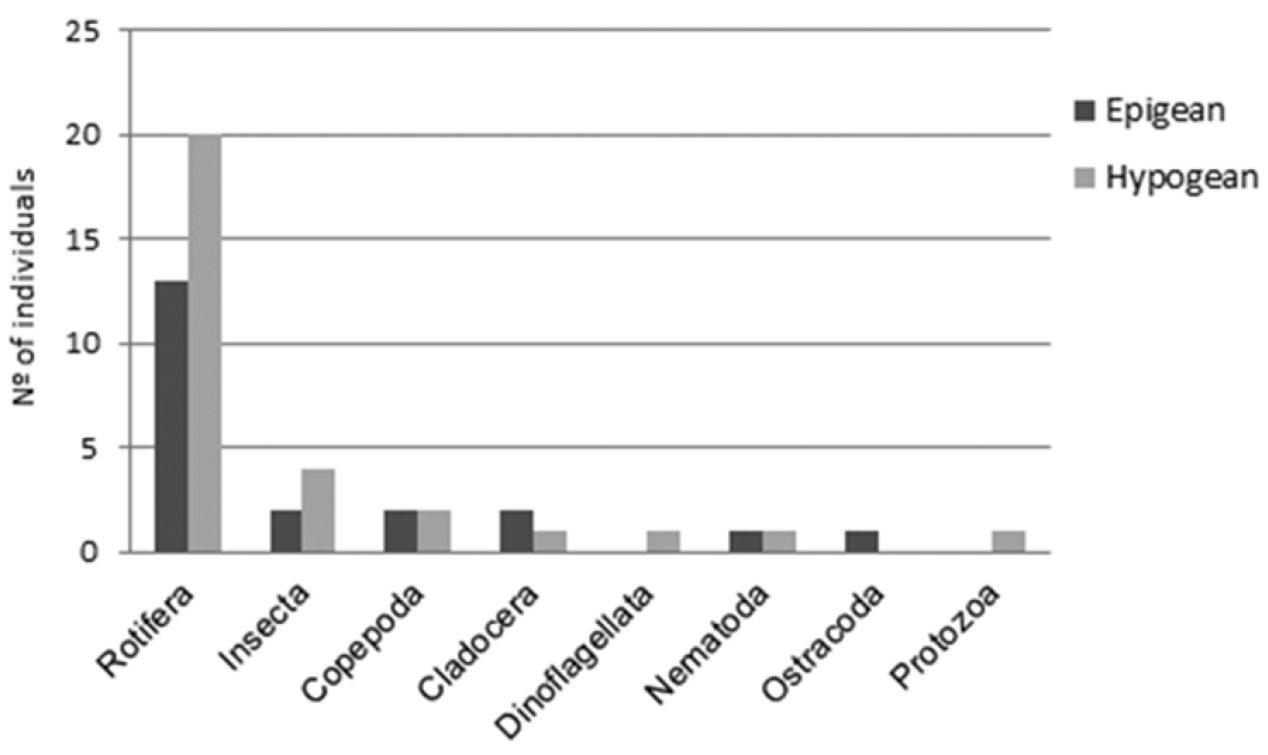
Thirty-six taxa were registered in all of the samples, belonging to different groups listed in decreasing order according to absolute/relative richness: Rotifera (23; 64%), Insect larvae (4; 11%), Copepoda-Cyclopoida, Calanoida and Harpacticoida (3; 8%), Cladocera (2; 5%), Dinoflagellata (1; 3%), Nematoda (1; 3%), Ostracoda (1; 3%) and Protozoa (1; 3%) (Table 2).
Taxa list and number of individuals sampled in each point. Caves: Angélica (Ang), Buraco das Araras (BAra), Bezerra (Bez), São Bernardo (SBer), São Mateus (SMat), Revolucionários (Rev); epigean rivers: Palmeiras (Palm), Angélica (Ang), São Domingos (SD); resurgences: Terra Ronca (RTR). Undet= undetermined taxa. Sampling point abbreviations are described in Table 1.
| Sampling points | Drips | Pools | Subterranean rivers | Epigean rivers | |||||||||||||
| Ang01 | Ang02 | BAra | Bez | Bara | Bez | SMat | Ang Ek | SBerEk | BezS | RevS | PalmEp | AngEp | BAraEp | SDEp | RTR | ||
| Taxa/ Sampled Volume (L) | 83 | 3 | 21 | 45 | 45 | 113 | 210 | 3, 802 | 2, 592 | 2, 603 | 1, 545 | 110 | 23, 580 | 6, 000 | 72, 000 | 158 | |
| Rotifera | 3 | ||||||||||||||||
| *Anuraeopsis fissa | 1 | ||||||||||||||||
| Anuraeopsis sp. | 1 | ||||||||||||||||
| Brachionus falcatus | 1 | ||||||||||||||||
| Brachionus sp. | 2 | ||||||||||||||||
| Chonochilidae | 2 | ||||||||||||||||
| Collotheca sp. | 4 | 4 | 2 | 1 | 2 | 2 | 3 | 18 | 15 | ||||||||
| Conochilus sp. | 1 | ||||||||||||||||
| *Euchlanis sp. | 12 | ||||||||||||||||
| Filinia sp. | 1 | ||||||||||||||||
| Gastropus sp. | 1 | 25 | |||||||||||||||
| Hexarthra sp. | 1 | 1 | |||||||||||||||
| Kellicotia bostoniensis | 5 | ||||||||||||||||
| Keratella cochelaris tecta | 4 | ||||||||||||||||
| Keratella cochelaris | 4 | 1 | 1 | 2 | 2 | 4 | |||||||||||
| Lecanidae | 3 | 2 | 2 | 18 | |||||||||||||
| Lecane sp. | 2 | 5 | |||||||||||||||
| Lecane monostyla sp. | 2 | 12 | |||||||||||||||
| Ploimida | 2 | 4 | 47 | ||||||||||||||
| Synchaeta sp. | 1 | 2 | 1 | 46 | |||||||||||||
| Testudinella sp. | 1 | 2 | |||||||||||||||
| Trichocerca sp. | 1 | 6 | 7 | ||||||||||||||
| Bdelloidea | 3 | 3 | 2 | 7 | 2 | 4 | 3 | 3 | 7 | 55 | |||||||
| Copepoda | |||||||||||||||||
| Cyclopoida adult | 3 | 1 | 1 | 3 | 5 | 1 | |||||||||||
| *Cyclopoida copepodito | 1 | 1 | 2 | 46 | |||||||||||||
| *Cyclopoida nauplius | 1 | 2 | 1 | 1 | 2 | 9 | 3 | 9 | |||||||||
| Calanoida | 4 | 2 | |||||||||||||||
| Harpacticoida | 2 | 4 | 2 | 1 | 1 | 2 | 1 | ||||||||||
| Cladocera | 2 | 2 | |||||||||||||||
| Bosmina sp. | 1 | ||||||||||||||||
| Dinoflagellata | |||||||||||||||||
| Peridinium sp. | 6 | 2 | |||||||||||||||
| Insecta | 1 | 2 | 2 | 2 | 2 | 6 | 3 | 1 | 1 | ||||||||
| Chironomidae | 1 | 4 | 4 | 4 | 7 | ||||||||||||
| Chaoboridae | 1 | ||||||||||||||||
| Simuliidae | 1 | ||||||||||||||||
| Nematoda | 5 | 8 | 2 | 1 | 4 | 2 | |||||||||||
| Ostracoda | 1 | ||||||||||||||||
| Protozoa | |||||||||||||||||
| Arcella costata | 1 | ||||||||||||||||
In decreasing order, the richest habitats (based on number of taxa present) were: AngEk (16); RTR (13); AngD02 (12); BAraP (10); BezS (9); PalmEp (8); AngD01, BAraD and AngEp (7); SBerEk, BezP, SMatP and RevS (5); SDEp and BAraEp (2); BezD (1). We summed up the richness of taxa from the samples of epigean and hypogean origin separately, totalling 21 and 30, respectively (Figure 4).
Figure 4. Number of taxa recorded in 16 sampling sites. Total taxa for epigean (21) and subterranean habitats (30).
Some taxa were exclusive to one single sampling point: Anuraeopsis fissa and Euchlanis sp. (RTR); Bosmina sp. (PalmEp); Anuraeopsis sp. (AngEp); Brachionus sp. (SBerEk); Chaoboridae (AngEk); Simuliidae (BAraP), Arcella costata (AngD01), Brachionus falcatus, Conochilus sp., Filinia sp., Kellicotia bostoniensis and Keratella cochlearis tecta (AngD02). The group Calanoida was restricted to samples with epigean origin, and Harpacticoida was restricted to subterranean waters.
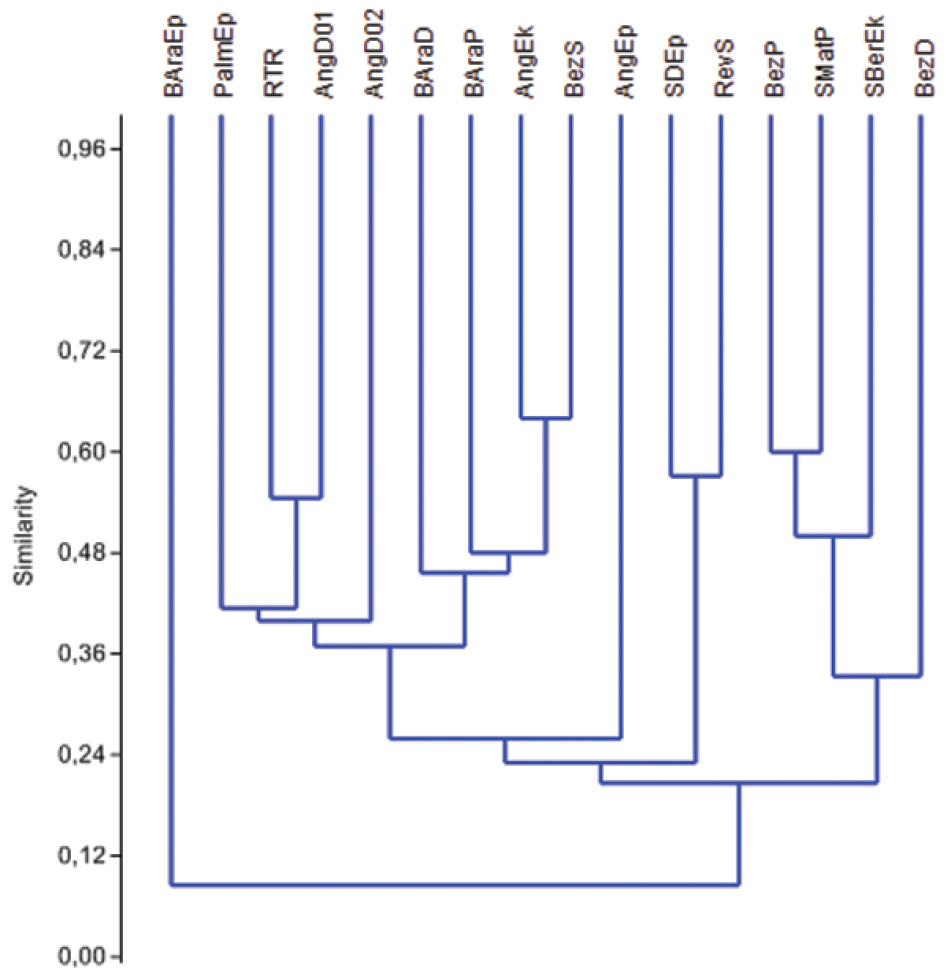
Cluster analysis for the 16 sites evidenced low similarity between them (S=0.32). The dendrogram also revealed that drips and epigean rivers are the most singular environments as they were more distant from other samples (Figure 5).
Figure 5. Similarity Cluster Analysis (Sorensen, single linkage) for 16 sampling sites from São Domingos karst area and surroundings, Goiás state, central Brazil. Abbreviations are described in Table 1.
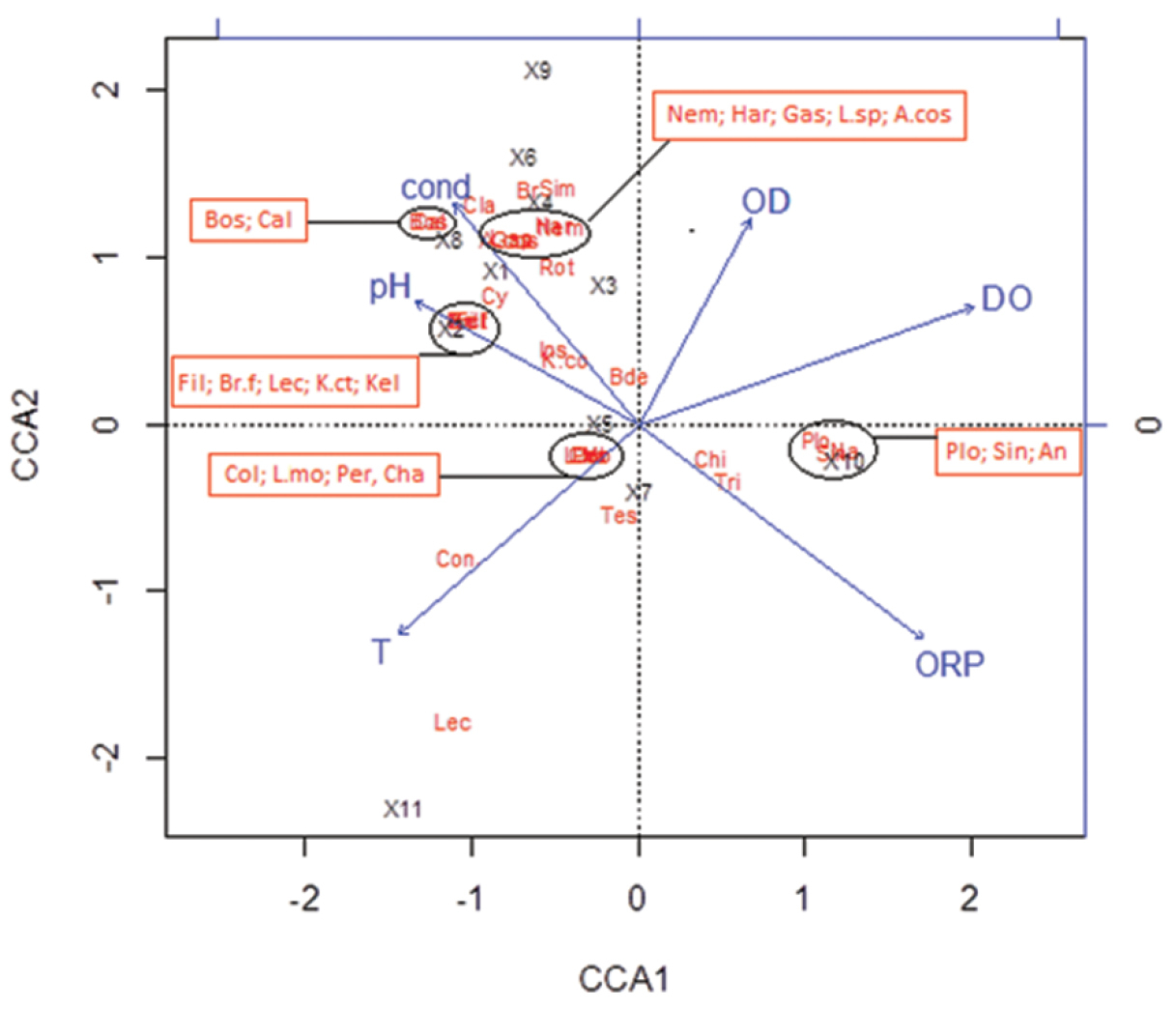
In the resultant CCA (Figure 6), the taxa closest to the various environmental vectors are strongly associated with them. For this analysis, thirty-one taxa were recorded in 11 sampling points. The majority of hypogean taxa are close related to both high levels of conductivity and pH, and low ORP. The epigean taxa appear more related to high levels of OD and ORP, except for the SDEp site. In the same way, taxa near each other (Figure 6) have similar environmental preferences. The sampling point positions reflect the physical and chemical characteristics of each habitat.
Figure 6. CCA for environmental variables and biotic data of 11 sampling sites from São Domingos karst area and surroundings, central Brazil (ORP= redox potential; T = temperature; OD= concentration of O2; DO = oxygen saturation and cond=conductivity). Red abbreviations represent the taxa: Rot = Rotifera; An = Anuraeopsis sp.; Br.f = Brachionus falcatus; Br= Brachionus sp.; Col = Collotheca sp.; Con = Conochilus sp.; Fil = Filinia sp.; Gas = Gastropus sp.; Kel= Kellicotia bostoniensis; K.ct= Keratella cochelaris tecta; K.co = Keratella cochelaris; Le c= Lecanidae; L.sp = Lecane sp.; L.mo = Lecane monostyla sp.; Plo = Ploimida; Sin = Synchaeta sp.; Tes = Testudinella sp.; Tri = Trichocerca sp.; Bde = Bdelloidea; Cy= Cyclopoida; Cal = Calanoida; Har = Harpacticoida; Bos = Bosmina; Cla = Cladocera; Per = Peridinium sp.; Ins = Insecta; Chi = Chironomidae; Cha = Chaoboridae; Sim = Simuliidae; Nem = Nematoda; A.cos = Arcella costata. X words represent the sampling sites (described at Table 1): X1=AngD01; X2=AngD02; X3=BAraD; X4=BAraP; X5=AngEk; X6=SBerEk; X7=BezS; X8=PalmEp; X9=BAraEp; X10=AngEp and X11=SDEp.
Only six of the ten environmental variables that were measured held some importance in the distribution of the samples according to fauna: dissolved oxygen, oxygen saturation, redox potential, conductivity, pH and temperature (Table 3). Oxygen saturation and redox potential presented the highest correlation values with the two first ordination axes (0.7802 and 0.5588, respectively). The two first canonical axes represented 51% of the total variation (30% and 21%, respectively).
Physical-chemical variables of the 11 sampling points in São Domingos karst area and surroundings, central Brazil. Cond=conductivity, OD=concentration of O2, OD=saturation of O2, ORP= redox potential, T=temperature. Sampling points abbreviations are described in Table 1.
| Sampling point | pH | Cond (mS/cm) | OD (mg/L) | DO (%) | ORP (mv) | T (°C) |
|---|---|---|---|---|---|---|
| AngD01 | 7.40 | 0.81 | 3.17 | 37.92 | 179.00 | 23.00 |
| AngD02 | 9.80 | 0.47 | 4.25 | 50.50 | 24.30 | 22.60 |
| BAraD | 8.20 | 0.49 | 12.80 | 145.90 | 210.20 | 22.30 |
| BAraP | 8.10 | 0.54 | 15.60 | 170.50 | 220.00 | 22.00 |
| AngEk | 7.50 | 0.49 | 4.48 | 102.63 | 215.90 | 23.90 |
| SBerEk | 6.30 | 0.45 | 3.50 | 42.73 | 203.90 | 24.50 |
| BezS | 7.50 | 0.09 | 5.55 | 66.03 | 300.00 | 23.00 |
| PalmEp | 7.60 | 0.01 | 6.03 | 75.75 | 68.70 | 26.20 |
| BAraEp | 7.00 | 0.02 | 11.55 | 197.70 | 204.40 | 25.30 |
| AngEp | 6.70 | 0.01 | 6.96 | 163.09 | 331.60 | 22.90 |
| SDEp | 7.00 | 0.02 | 2.91 | 36.80 | 20.00 | 26.50 |
Pearson correlation did not show a significant relation between the volume of water filtered and the number of taxa recorded at each sampling point, since simple linear regression analysis presented a value of R= 0.10756.
In this preliminary approach, we studied the richness of taxa in habitats of karstic systems, which represents new data on tropical subterranean domain species. The fauna belonging to samples of percolating water were remarkable and responsible for the high number of recorded species in the studied habitats
Rotifers were the richest group in our study, representing more than half of the total richness recorded. The dominance of rotifers was associated with the increase in the trophic state of a system due to short life cycles and rapid reproduction, which favour this group in more dynamic, competitive and selective environments. Presently, it is known that rotifers are also dominant in several other aquatic environments, independent of the trophic state (
Diversity in subterranean water courses is frequently limited by competition, and especially limited by energetic resources and space (
The low number of Copepoda occurrences in our samples must be due to environmental factors and/or interspecific relationships in the communities. In dry periods, the water volume flowing through the system can decrease, as well as the access to aquatic communities from unsaturated zone habitats. In fact, some of our sampling points could not be efficiently accessed in all periods, in part because of the reduced local rain rates. Besides, sampling frequency could have also affected our results to the extent that it did not offer access when abundant taxa of these communities occurred (i.e. different life cycles). We also have to consider that benthonic fauna was not well assessed.
The fauna distribution depends on the scale of the karst system and generally species change in number from the surface to the saturated zone (
We observed that drips samples have the most unique fauna of all the other subterranean habitats. In our study, it is important to highlight that the water volumes filtered in drips were much smaller than the volume of water filtered in other habitats. However, we encountered elevated richness and occurrence of taxa exclusive to these samples. Thus, we recorded the greatest richness of taxa from the least voluminous samples (drips of Angélica Cave and epigean resurgence), while some of the most voluminous ones had inferior richness (São Domingos, Lapa and Angélica epigean rivers). The total of taxa encountered in drips outnumbered all others samples, emphasizing the complexity of communities from the unsaturated zones.
For the environmental parameters considered, we have observed that there was a separation between epigean and hypogean sites in the diagram generated by CCA as well as in the dendrogram of faunistic similarity (except for SDEp and RevS sites). Both demonstrated that there were clear differences between the biota of epigean and groundwater environments while distinctions of hypogean habitats among themselves are less evident. When comparing each sample individually, we noticed faunistic distinction between them.
Epikarst and sinkholes are both responsible for supplying the aquatic hypogean realm, but they differ in their capacities to redirect and/or store water through the karst (
The waters that percolate the subterranean systems are filtered by soils on the karst surface and tend to suffer reduction of dissolved oxygen. Organic material, carbon, and diverse ions suffer reduction of dissolved oxygen, depending on the manner in which they are used and transformed through chemical processes by the interstitial and/or unsaturated zone biota. Water circulation in surface rivers promotes higher oxygenation rates than in the subterranean environment, which generally are formed by smaller and less dynamic or lentic systems (
Redox potential, the second most important variable in the analysis, has negative correlation to concentrations of oxygen, suggesting that reductive processes predominate in the absence of oxygen. Low levels of ORP can be associated with environmental pollution and/or high concentrations of ions, as occurs in waters with karstic origins (
Comparing the results obtained with studies on the unsaturated zone in temperate regions (
The presence of exclusive taxa at some sites emphasizes the singularity of the studied systems. There are faunistic differences between sites belonging to the same limestone lens and geographically neighbouring. Many species inhabiting the void network of the unsaturated zone are known to have a linear distribution of only a few hundred metres (
The structure of the unsaturated zone can vary significantly in different regions according to its lithology, geomorphic history, as well as environmental parameters and seasonal variables (
Nowadays, there is an incessant increase in agricultural, livestock and mining activities in many karst areas in Brazil. In the surroundings of the studied area, cattle and extensive plantations of soybean and cotton can trigger pollution and/or siltation of these streams. Besides, there are many land ownership problems and clandestine mining, which cause deforestation and endanger the integrity of the subterranean environments.
The richness and distribution of aquatic species vary dependent on water quality, and the unsaturated zone biota can be used as a bio-indicator for the impact of polluting actions (
To efficiently assess this particular fauna, it is necessary to constantly monitor the subterranean habitats by means of frequent sampling during different periods of the year. As such, not only can species richness be recorded but it also can be verified whether there are differences in their distributions and abundances according to seasonality. The use of combined sampling methods (e.g. micrometric mesh nets and vacuum pumps) is also recommended to efficiently access both the benthonic and planktonic fauna.
In Brazil, studies on diversity of aquatic biota in subterranean environments are few and data is still sparse (
We are grateful to colleagues at the Laboratório de Estudos Subterrâneos/UFSCar for helping in the field collections (C. S. Fernandes; D. M. Neto; J. E. Gallão; P. P. Rizzato) and also to N. Negreiros for taxa identification. Statistical analyses were performed with the aid of G. H. Carvalho. We are grateful to the two anonymous reviewers and the editor for valuable suggestions which improved the manuscript and to Maria-Ana Laza for the English correction. Logistics were made possible thanks to the financial support of FAPESP to M. E. Bichuette (process nº 2010/08459-4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), through a master’s grant awarded to the first author (#132981/2011-4). All of the collections was performed in agreement with Brazilian state (authorization for scientific research in the conservation unit SEMARH nº 063/2012) and federal laws (SISBIO # 28992-1). We also thank PPGERN/UFSCar for the infrastructure used in the present study.