(C) 2013 Alexander M. Weigand. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Weigand AM (2013) New Zospeum species (Gastropoda, Ellobioidea, Carychiidae) from 980 m depth in the Lukina Jama–Trojama cave system (Velebit Mts., Croatia). Subterranean Biology 11: 45–53. doi: 10.3897/subtbiol.11.5966
A new species of the eutroglobiont gastropod taxon Zospeum Bourguignat, 1856 is described. Zospeum tholussum sp. n. is characterized based on a population from the Lukina Jama–Trojama cave system (Velebit Mts., Croatia). A single living specimen occurred at 980 m depth. The species is morphologically related to Zospeum amoenum (Frauenfeld, 1856), but can be readily distinguished from the latter by the presence of a weak columellar fold and its dome-like structured 2nd whorl. DNA barcoding is capable to clearly delineate Zospeum tholussum from other Zospeum spp. as well.
DNA barcoding, cryptic species, biospeleology, eutroglobiont gastropod, cave-dwelling species, microgastropoda
Taxonomic research distinguishes eleven morphospecies (no subspecies considered) of the microgastropod taxon Zospeum Bourguignat, 1856 (Ellobioidea, Carychiidae) for the region of the Dinaric Alps (
All known Zospeum have lost visual orientation and are considered true eutroglobionts (see
Here, a new Zospeum population from the Lukina Jama–Trojama cave system situated in the Velebit mountain range of Croatia is characterized (Fig. 1). On the basis of molecular and conchological data, a new species from 980 m depth–Zospeum tholussum Weigand, sp. n.–is described.
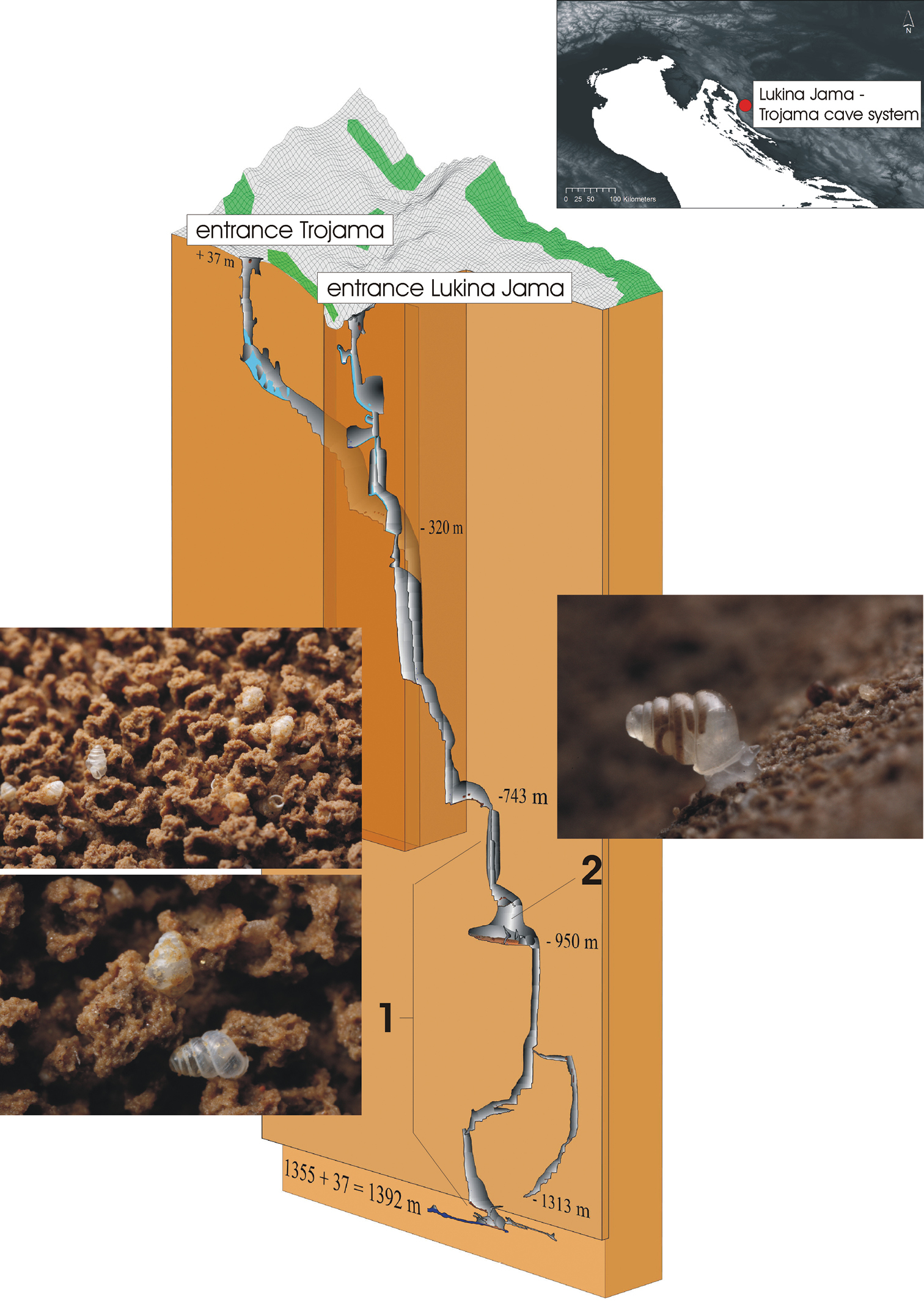
The Lukina Jama–Trojama cave system. Overview of the geographical position and 3D cave cross-section. In the latter, the region of collected shells (1) and the collection site of the living specimen of Zospeum tholussum (2) are indicated. The 3D cross-section was provided by D. Bakšić et al. (2010), Croatian Speleological Server, http://www.speleologija.hr/lukinajama. Photos were taken by J. Bedek.
Specimen shells were measured by taking individual images with 456 pixels corresponding to 1 mm. The total shell height (SH), shell width (SW), aperture height (AH), aperture width (AW) and the number of whorls were measured. Moreover, ratios for SH/SW and AH/AW were calculated.
Specimen shells of live-collected Zospeum tholussum sp. n. (single specimen) and Zospeum amoenum (Frauenfeld, 1856) (two specimens) were kept intact by using a protocol for a non-invasive DNA isolation method originally described in
PCR conditions were the same as described in
DNA barcodes, chromatograms, images, geographic data and further information of the genetically analysed Zospeum tholussum (BOLD-ID BARCA210-13) and Zospeum amoenum (BOLD-IDs BARCA211-13 and BARCA212-13) specimens are deposited in the Barcode of Life Database (BOLD) (
http://zoobank.org/A3B380AD-B918-451B-866D-7FA59642A733
http://species-id.net/wiki/Zospeum_tholussum
Figs 1–4A single living specimen was collected on 31.07.2010 (leg. Jana Bedek), which is designated as the holotype specimen (BOLD-ID BARCA210-13) (Fig. 1, life image; Fig. 2, upper row). Eight shells comprise paratype specimens, of which one was partially broken and used to investigate the form of the columellar fold. Shells were collected on several days during the caving expedition from 29.07. - 03.08.2010 (Fig. 2). All investigated specimens are deposited in the Forschungsinstitut und Naturmuseum Senckenberg, Frankfurt am Main, Germany (museum voucher SMF 341633).
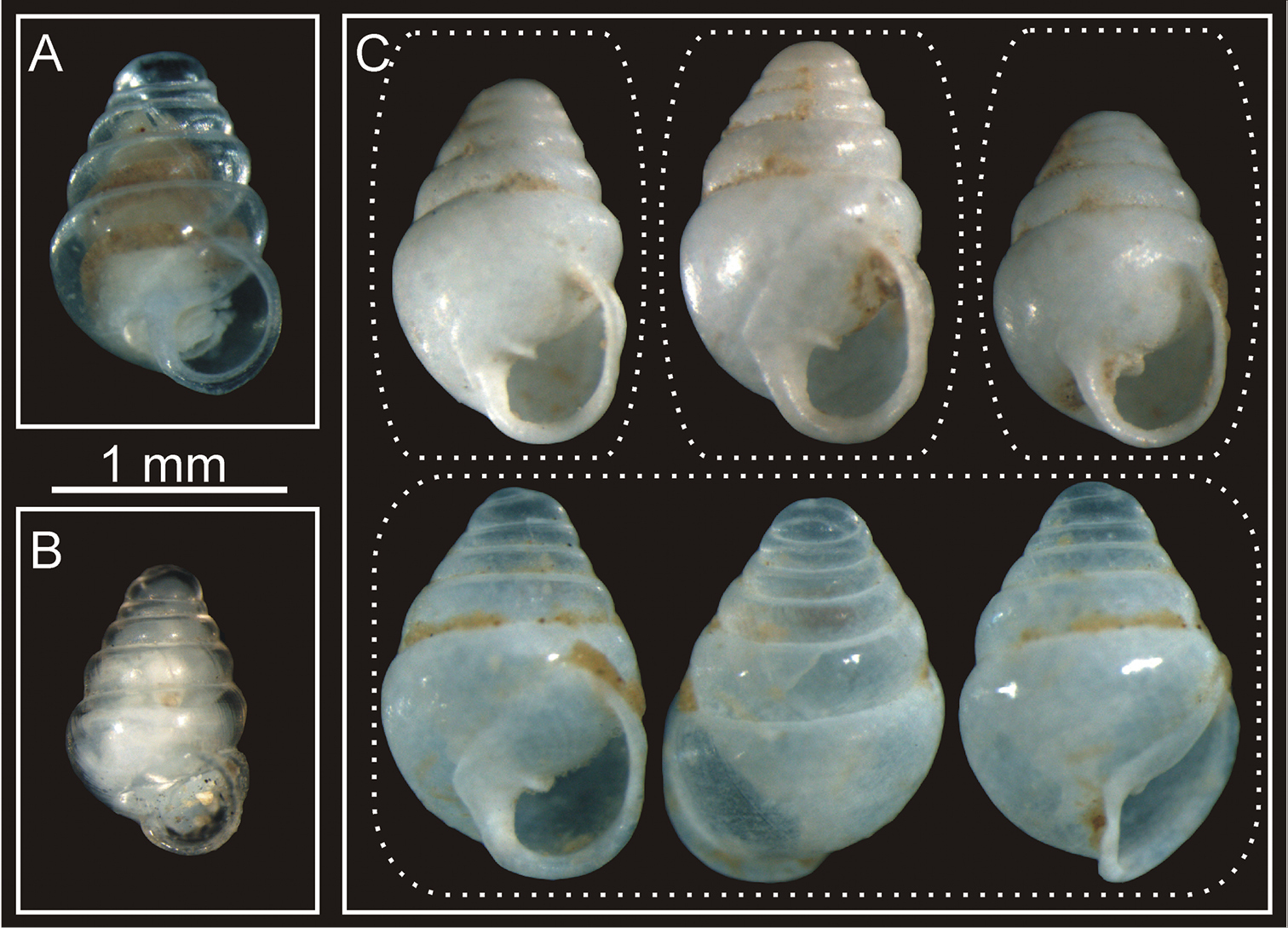
Holotype and paratypes of Zospeum tholussum. The holotype (former living specimen) is marked with a solid line; five paratype specimens (shells) are surrounded by dotted lines.
The general shape of Zospeum tholussum sp. n. resembles larger specimens of Zospeum amoenum (Frauenfeld, 1856) from which it can be best delineated by the dome-like structured 2nd whorl (Fig. 2), the weak but present columellar fold (Fig. 3) and by means of their DNA barcodes (11.7 %–12.1 % genetic p-distance between both species).
The eight conchologically investigated specimens (the holotype and seven paratypes) demonstrate moderate shell variability (Table 1). Shells are generally smooth, very thin and fragile. Fresh shells or those of living specimens are translucent, older ones adopt a milky white color. The number of whorls are 5–6, SH ranges from 1.41–1.81 mm, SW from 0.93–1.12 mm, AH from 0.44–0.54 mm and AW from 0.38–0.46 mm. The ratio of SH/SW is between 1.34–1.62 and AH/AW between 1.05–1.30. The 2nd whorl is remarkably enlarged possessing a height of 2/3–5/6 the height of the 3rd + 4th whorl. The columellar is only weakly developed (Fig. 3) and no teeth (i.e. parietalis, palatalis or columellaris) are present. The DNA barcode of the holotype, when compared with other DNA barcodes of Zospeum species deposited in BOLD, shows its lowest interspecific genetic distance to Zospeum pretneri Bole, 1960 (5.6 %; BOLD-ID: BARCA120-10). This result is well above the barcoding gap of 3.2 % for Carychiidae, which is suitable to separate between-species (> 3.2 %, interspecific) and within-species genetic diversity (< 3.2 %, intraspecific) in Zospeum (
Conchological measurements for Zospeum tholussum. Seven paratypes (Ind. 1–7) and the holotype specimen (Ind. 8) were measured. All measurements are given in millimeter. Additionally, ratios of shell height / shell width (sh/sw) and aperture height / aperture width (ah/aw) were calculated. The range of variability is given for all parameters.
| Ind. | sh/sw | ah/aw | shell height | shell width | aperture height | aperture width |
|---|---|---|---|---|---|---|
| 1 | 1.60 | 1.16 | 1.49 | 0.93 | 0.44 | 0.38 |
| 2 | 1.42 | 1.30 | 1.54 | 1.08 | 0.52 | 0.40 |
| 3 | 1.46 | 1.16 | 1.41 | 0.97 | 0.46 | 0.39 |
| 4 | 1.34 | 1.05 | 1.44 | 1.07 | 0.47 | 0.44 |
| 5 | 1.50 | 1.31 | 1.63 | 1.09 | 0.54 | 0.41 |
| 6 | 1.53 | 1.23 | 1.61 | 1.05 | 0.51 | 0.41 |
| 7 | 1.62 | 1.15 | 1.81 | 1.12 | 0.53 | 0.46 |
| 8 (holotype) | 1.48 | 1.12 | 1.47 | 0.99 | 0.44 | 0.39 |
| range | 1.34–1.62 | 1.05–1.30 | 1.41–1.81 | 0.93–1.12 | 0.44–0.54 | 0.38–0.46 |
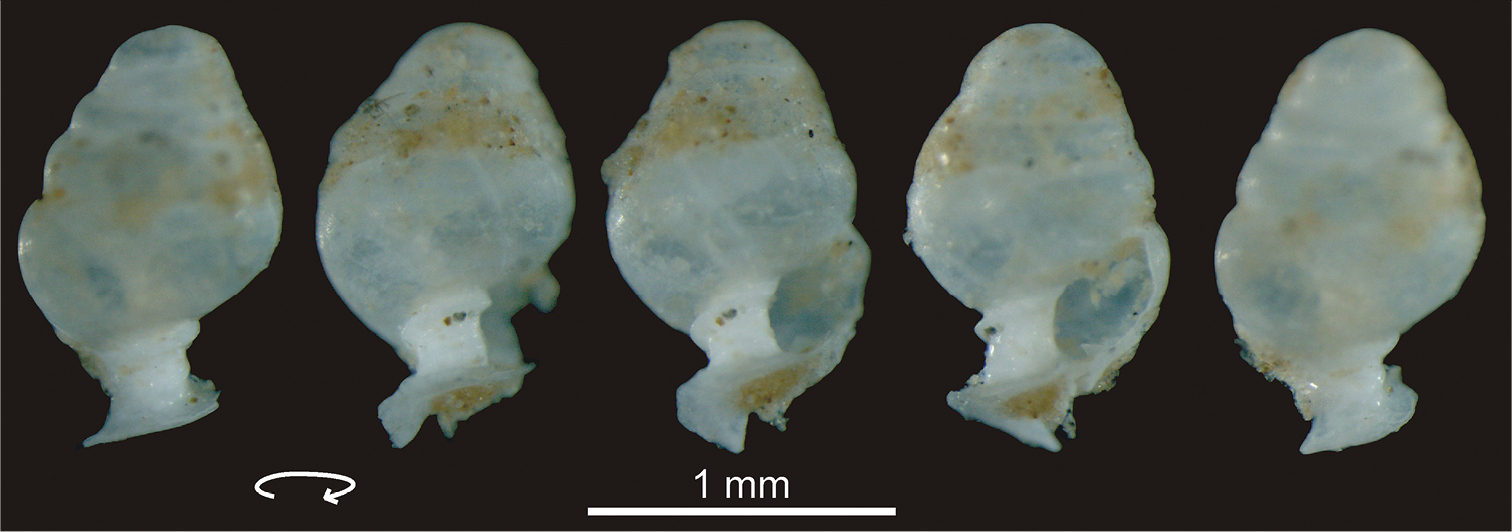
Columellar fold views of Zospeum tholussum. The columellar fold of the broken paratype specimen shell is shown in clockwise rotation.
The single living specimen was found in an unnamed large chamber at 980 m depth (85 m long, 70 m wide) with lots of stones, rocks and sand (Fig. 1, right site). A temporal small stream of running water was present close to the collecting site. Air temperature was between 3.3–3.5 °C (depending on the measurement device), water temperature 5.1 °C and air humidity 100 %. Shells were observed beginning from 800 m depth till the bottom of the cave. Shells were generally found on layers of mud (Fig. 1, left site). The first 200 m of the entrance passage of Lukina Jama are permanently covered by varying levels of snow and ice.
The Latin word tholus means dome or cupola and refers to the remarkable dome-like shape of the 2nd whorl.
In addition to the newly described Zospeum tholussum, a second Zospeum species is present in the Lukina Jama–Trojama cave system (Fig. 4C). This species can be differentiated from Zospeum tholussum by the presence of a tooth, its general shell shape, more prominent columellar fold and absence of the characteristic dome-like structured 2nd whorl. Because only shells were found, no DNA barcodes are available for this species.
Zospeum species. A Zospeum tholussum holotype B Zospeum pretneri from locus typicus, which demonstrates the lowest genetic distance to Zospeum tholussum C Second Zospeum sp. from the Lukina Jama–Trojama cave system.
So far, Zospeum tholussum is only known from the Lukina Jama–Trojama cave system. However, this cave system is situated in the distribution range of the morphologically related species Zospeum amoenum (Frauenfeld, 1856), which inhabits caves of the West Balkan of North Slovenia, West Croatia, Bosnia and Herzegovina and South to Montenegro (
The preliminary information on the habitat of Zospeum tholussum is in congruence with previous findings for Zospeum. Interestingly, the grazing-labyrinth-like structure, in which most of the shells were embedded (Fig. 1, left site), has been already observed during caving expeditions in Northern Spain (
I thank Jana Bedek and all members of the HBSD (Zagreb, Croatia) for their permanent biospeleological effort and the samples, Adrienne Jochum (Frankfurt, Germany) and Rajko Slapnik (Ljubljana, Slovenia) for their taxonomic assistance and Yasunori Kano (Tokyo, Japan) for his introduction to the method of non-invasive DNA isolation. The research on the adjustment of this method to carychiid microgastropods carried out at the AORI (University of Tokyo, Japan) was supported by the Japan Society for the Promotion of Science (JSPS). The 3D cross-section of the Lukina Jama–Trojama cave system was kindly provided by D. Bakšić et al. (2010), Croatian Speleological Server, http://www.speleologija.hr/lukinajama. The collecting permit for the Croatian Biospeleological Society (HBSD) was issued by the Croatian Ministry of Culture, dated 24 June 2010, no. 538-08-01-01/3-10-02.