(C) 2013 Akos Fejér. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Drimeotus viehmanni (Coleoptera, Leiodidae) is abundant in the cave Peştera cu Apă din Valea Leşului (Western Carpathians) and was chosen for a mark-release-resight experiment. The aims of the experiment were to estimate the size of the population and to analyze the dispersal patterns inside the cave, for conservation purposes. During the three years’ study, the observed abundance of Drimeotus viehmanni was significantly higher in summer compared to the winter season. The seasonal dynamics can not be explained by climate features such as temperature and air relative humidity which had low or no variation during all seasons. Few marked beetles were re-seen during the mark-resight experiment proving the existence of an important cave/subterranean population, which was estimated between 5, 084 and 533, 033 individuals. The marked individuals moved between neighbouring patches on a distance of 10 m over the same amount of time as on distances longer than 200 m. Dispersal inside the cave occurs during the winter months, which indicates non-continuous behaviour triggered by environmental features and involving only a negligible part of the population in the studied cave.
Cave beetles, population dynamics, mark-resight, migration, cave climate, Carpathians, Romania
Estimation and prediction of population sizes have been a challenge and a must of many ecological studies (
Although the total number of cave species has been estimated to 50, 000-100, 000 (
The use of the mark-recapture methods to evaluate subterranean beetle populations is problematic due to the difficult access to populations, and both long life and low reproduction rates of their representatives (
The aim of our study was to analyse the patterns of distribution for a beetle population inside one cave and in correlation to topoclimate variations, and to estimate the cave population size by using the mark-resight method. The results on both population size and dispersal behaviour of a subterranean beetle population are the starting point in further studies about the impact of the touristic infrastructure on cave inhabitants.
We chose Drimetous s. str. viehmanni (Ienistea, 1955) (Coleoptera, Leiodidae, Leptodirini) from the cave Peştera cu Apă din Valea Leşului for the mark-resight experiment. Drimeotus s. str. endemic for the Pădurea Craiului Mountains (North-Western Romania) is represented by 14 species most of them endemic for one cave system or a complex of caves in one valley (
The study on the cave population of Drimeotus viehmanni was carried out in the Peştera cu Apă din Valea Leşului, between March 2005 and Novembe
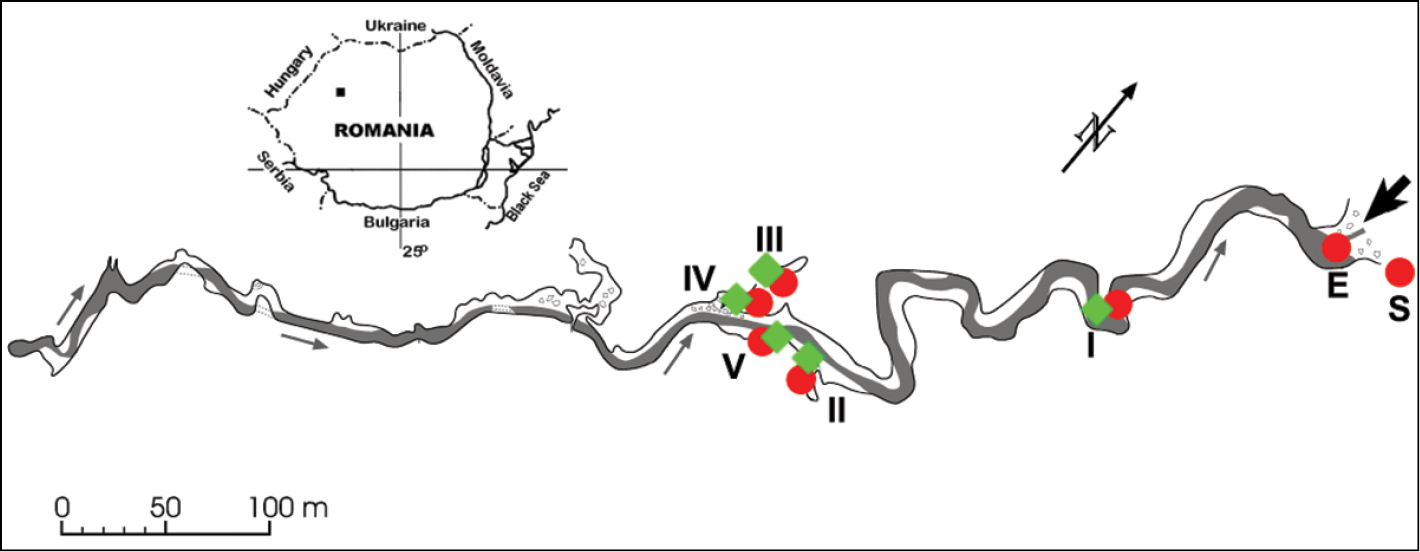
The Peştera cu Apă din Valea Leşului is located in the eastern part of the Western Carpathians (North-Western Romania) at an altitude of 700 m a.s.l. The cave has one main gallery in total length of 1265 m (
Peştera cu Apă din Valea Leşului (modified after
The cave microclimate is typical for a horizontal cave with one entrance; two different air flow systems, caused by temperature differences between cave and surface, are influencing the winter and the summer cave climate (
A mark-release-resight method was used for the estimation of the population abundance (N). During the first counting, every individual elytra was marked with non-toxic paint (as described in
N = M (n + 1) / m + 1
where: N = the actual size of the study population, M = the number of individuals marked and released, m = the number of marked individuals in a sample of the population, n = the total number of individuals in the sample.
The mark-resight method is different from the traditional mark-recapture methods, in that it includes data on encounters of marked individuals and of the unmarked individuals. Individuals are marked prior to sampling which consists of sighting surveys instead of capture periods. The main advantage of this approach is that it is less invasive than the traditional mark-recapture (
328 individuals were marked the first time and 1065 individuals the second time. The individuals were marked in March 2005 at station II and in July 2005 at station III. Two different colours have been used, as follows: yellow at station II and blue at station III. The two points are on the opposite banks of the subterranean stream.
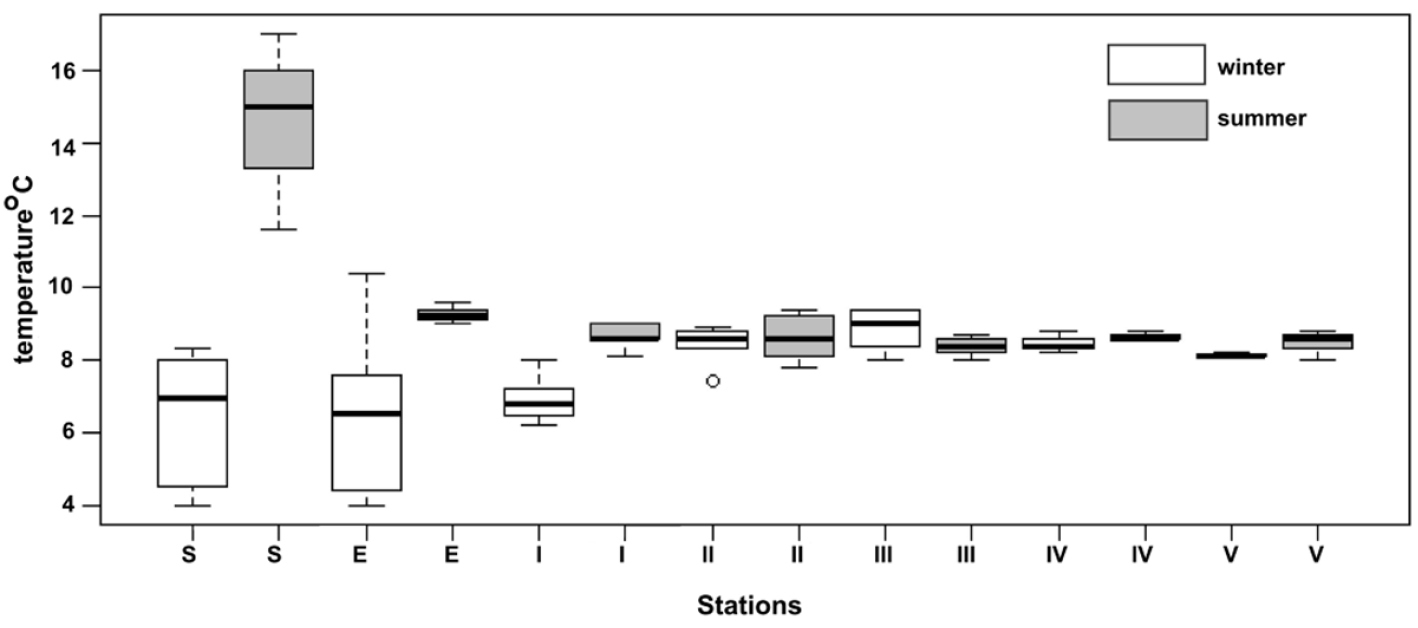
The amplitude of the air temperature in the Peştera cu Apă din Valea Leşului decreases inwards the cave, from 10.5°C at the surface to 8.3°C at station V (both are mean values for the period October 2006-Novembe
Box-plot of the air temperature, during winter and summer months, at the surface (S), the entrance (E) and the five stations (I-V) for Peştera cu Apă din Valea Leşului.
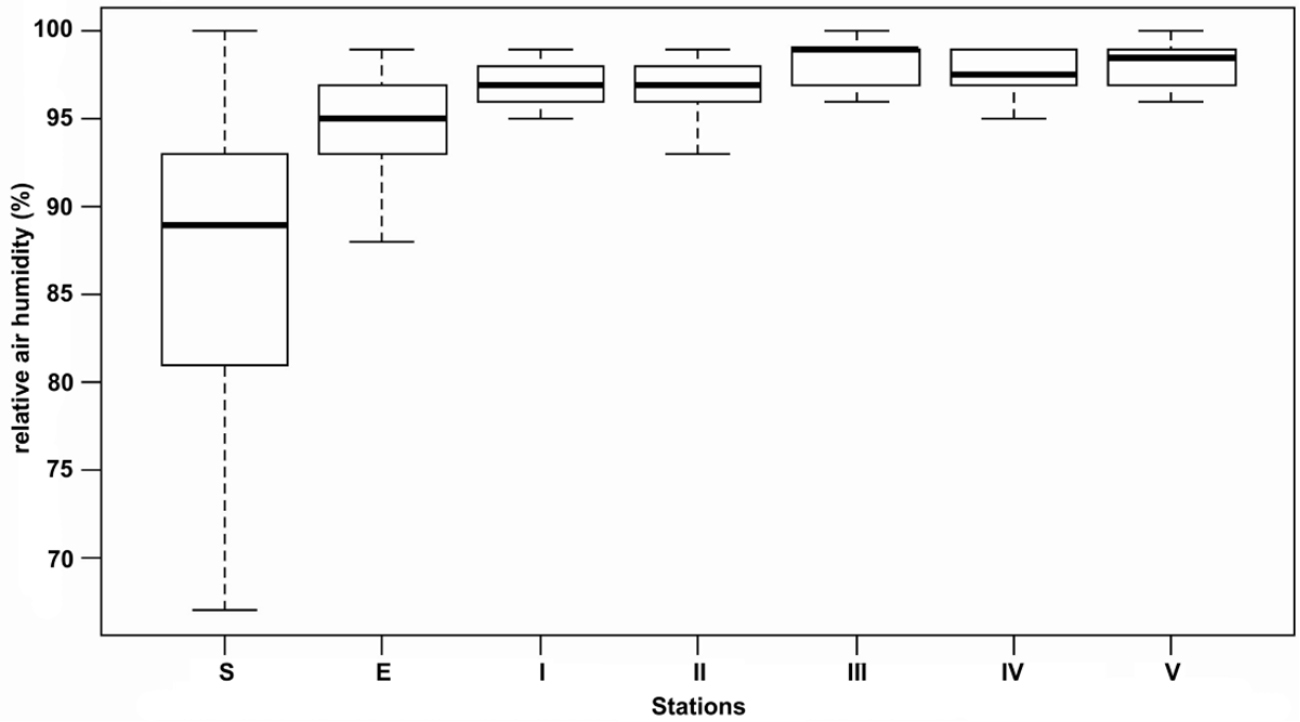
Box-plot of the relative air humidity at the surface (S), the entrance (E) and the five stations (I–V) for Peştera cu Apă din Valea Leşului.
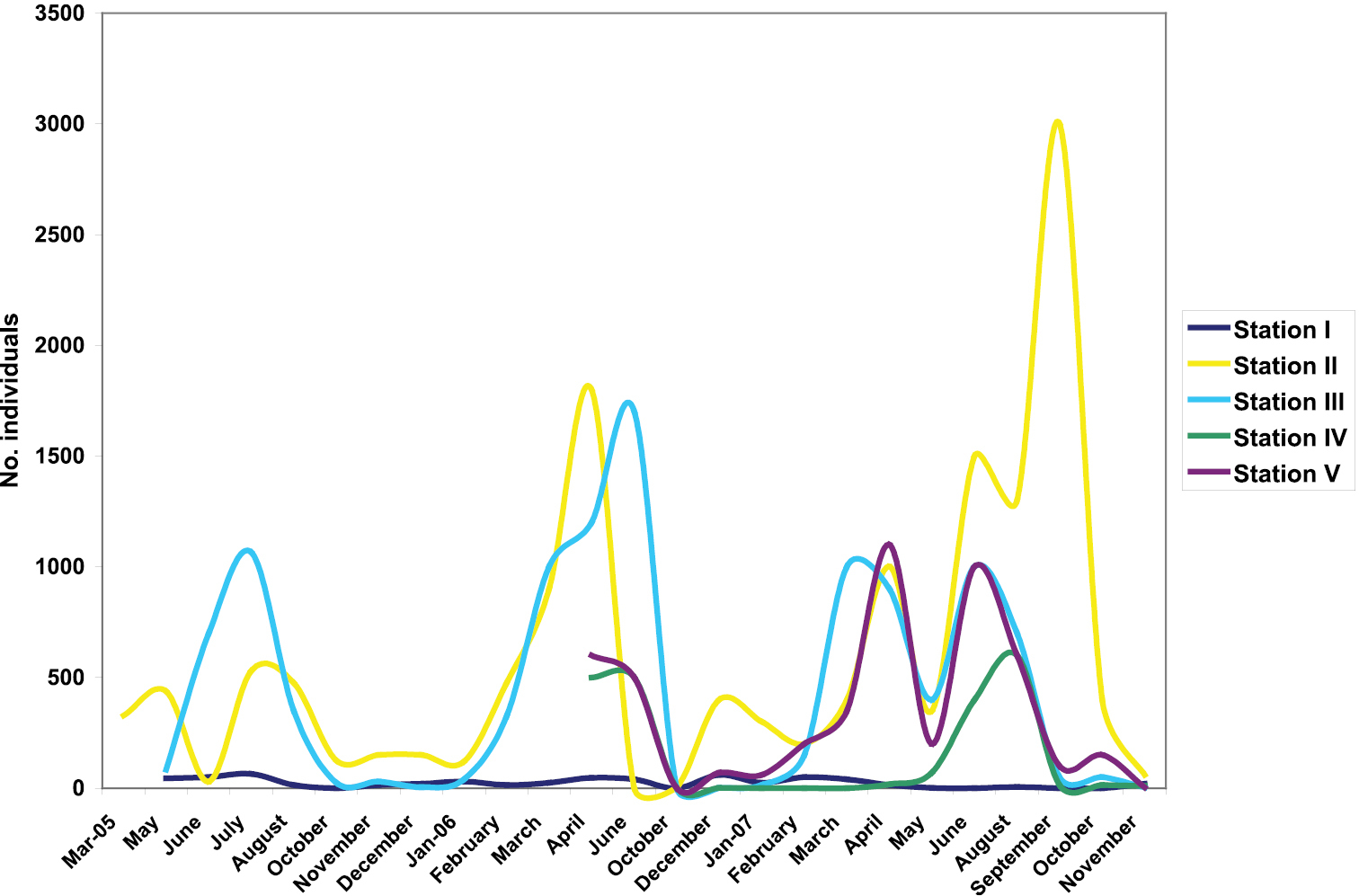
During the study, the highest abundances of Drimeotus viehmanni were recorded at stations II and III (Fig. 4, Table 1). At station II, most individuals were counted in Septembe
Monthly variation of the number of individuals of Drimeotus viehmanni in the five stations (I–V) of Peştera cu Apă din Valea Leşului.
Total number and number of marked individuals of Drimeotus viehmanni from Peştera cu Apă din Valea Leşului, during the period March 2005 – Novembe
| Month | Station I | Station II | Station III | Station IV | Station V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Marked | Total | Marked | Total | Marked | Total | Marked | Total | Marked | |
| Mar-05 | 328 | 328 | ||||||||
| May | 45 | - | 437 | 12 | 83 | - | ||||
| June | 50 | - | 30 | 1 | 700 | - | ||||
| July | 65 | - | 530 | 10 | 1065 | 1065 | ||||
| August | 15 | - | 475 | 3 | 350 | 9 | ||||
| October | 0 | - | 125 | - | 25 | 1 | ||||
| November | 15 | - | 150 | - | 30 | - | ||||
| December | 20 | - | 150 | - | 5 | - | ||||
| Jan-06 | 30 | - | 120 | - | 40 | 2 | ||||
| February | 15 | - | 475 | 2 | 325 | 1 | ||||
| March | 25 | - | 900 | 1 | 1000 | 1 | ||||
| April | 47 | - | 1800 | - | 1200 | 2 | 500 | 1 | 600 | - |
| June | 40 | - | 0 | - | 1700 | - | 500 | - | 500 | - |
| October | 0 | - | 4 | - | 1 | - | 1 | - | 0 | - |
| December | 60 | 1 | 400 | - | 1 | - | 1 | - | 70 | - |
| Jan-07 | 25 | 1 | 300 | - | 15 | 1 | 0 | - | 60 | - |
| February | 50 | - | 200 | - | 150 | - | 0 | - | 200 | - |
| March | 40 | - | 400 | - | 1000 | - | 0 | - | 350 | - |
| April | 15 | - | 1000 | - | 900 | - | 18 | - | 1100 | - |
| May | 1 | - | 350 | - | 400 | - | 70 | - | 200 | - |
| June | 0 | - | 1500 | - | 1000 | - | 400 | 1 | 1000 | - |
| August | 6 | - | 1300 | - | 700 | - | 600 | - | 600 | - |
| September | 0 | - | 3000 | - | 50 | - | 15 | - | 100 | - |
| October | 0 | - | 400 | - | 50 | - | 15 | - | 150 | - |
| November | 20 | - | 60 | - | 3 | - | 10 | - | 3 | - |
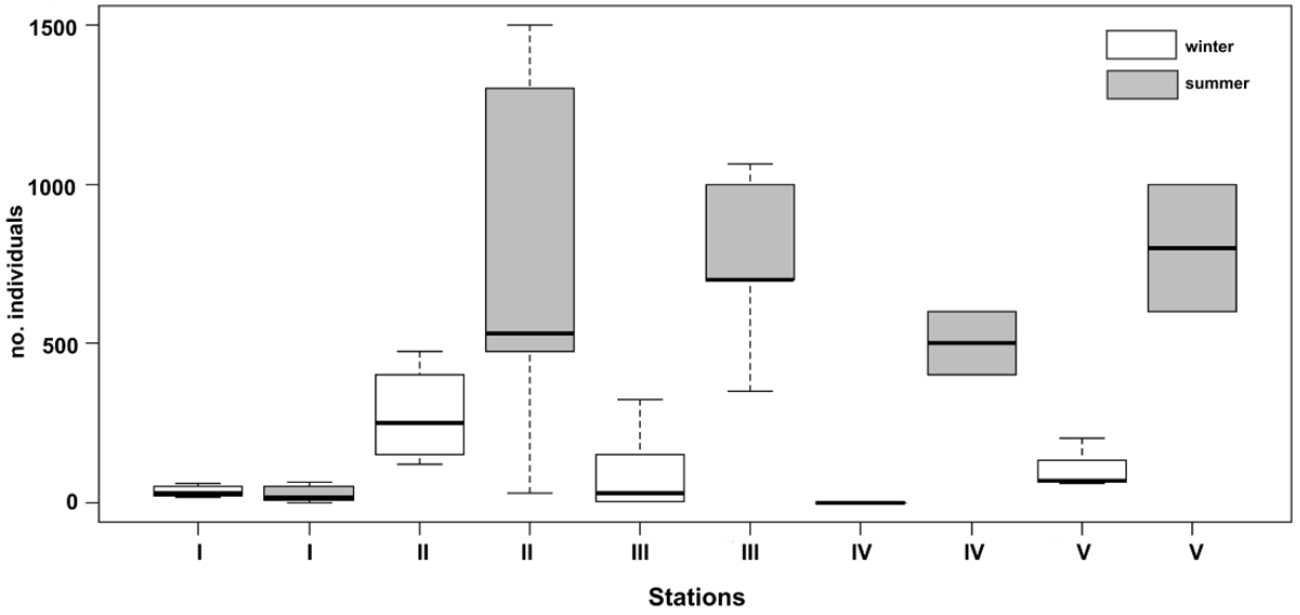
When comparing the number of individuals, a significant difference between the five stations (KW: chi2 = 30.2, df = 4, p < 0.001) can be noticed (Fig. 5). Because station I had a low number of beetles, we compared only the other four stations, but the difference remained significant (KW: chi2 = 8.9, df = 3, p = 0.03).
Box-plot of the number of individuals of Drimeotus viehmanni, in the winter and the summer months, in the five stations (I–V) of Peştera cu Apă din Valea Leşului.
For a better understanding of the seasonal behaviour of the studied species, we separated the data recorded in winter from the data recorded during the summer months (Fig. 5). The results show an important difference between the two seasons in all the stations, except station I where more individuals were recorded in the winter months (MW = 101, p = 0.002).
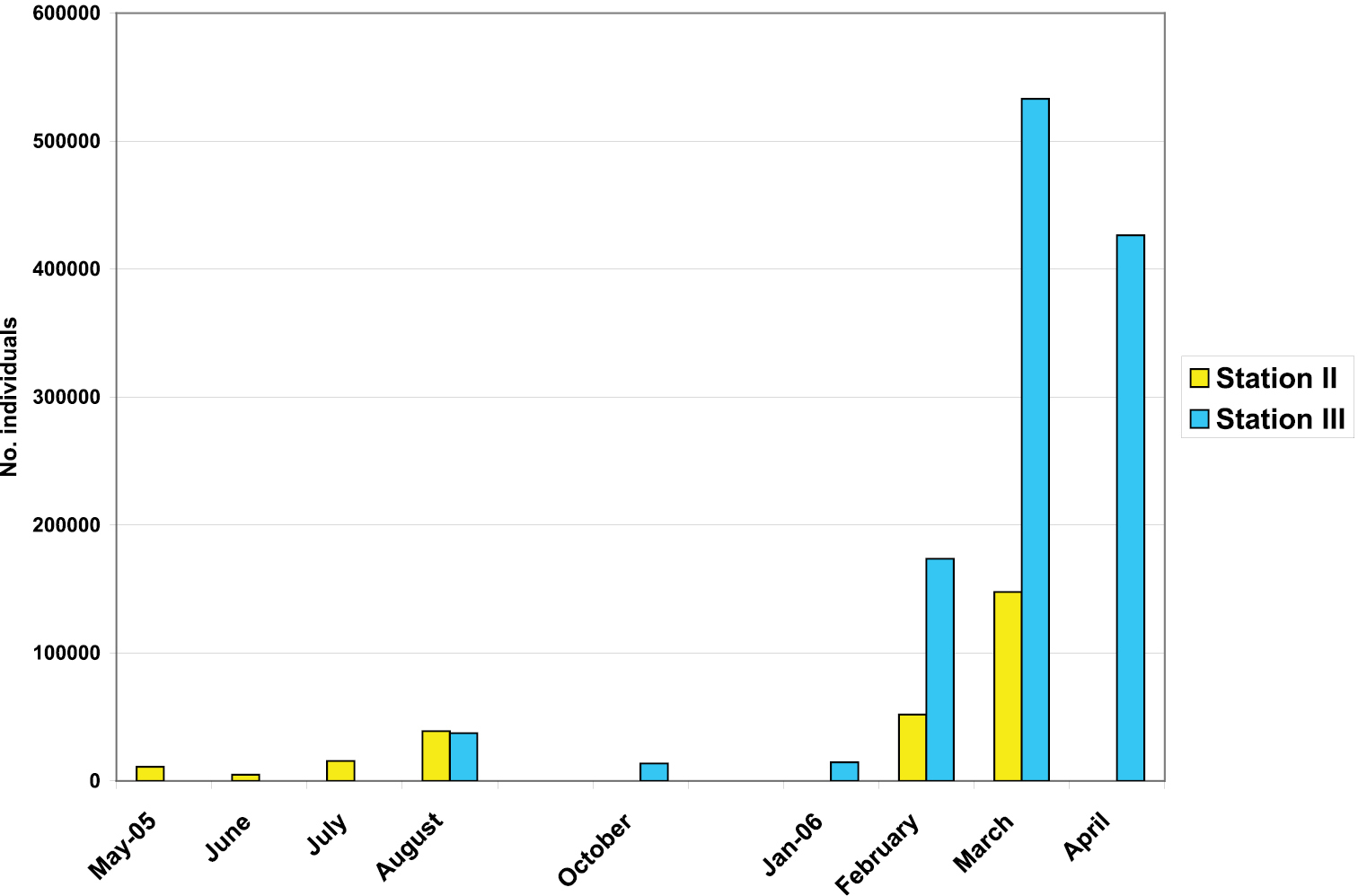
Estimates of the population size in the Peştera cu Apă din Valea Leşului were obtained from the mark-resight data (Table 1). At station II, from the 328 individuals marked in March 2005, the highest number of recaptured individuals was 12, in May 2005. The re-seen marked individuals decreased from month to month and hereby in June 2005 there were 11 beetles marked from the total of 30 observed beetles. According to these data the estimated population size at station II varied between 5, 084 and 147, 764 (Fig. 6).
The evolution of the Drimeotus viehmanni abundance in two stations of Peştera cu Apă din Valea Leşului.
At station III, from the 1, 065 marked beetles most individuals were observed in the next month (9 marked individuals). Afterwards, there were just one or two marked beetles at the bait. With the Lincoln-Petersen formula the lowest estimated size of the population at station III was of 13, 845 and the highest was of 533, 033 individuals (Fig. 6).
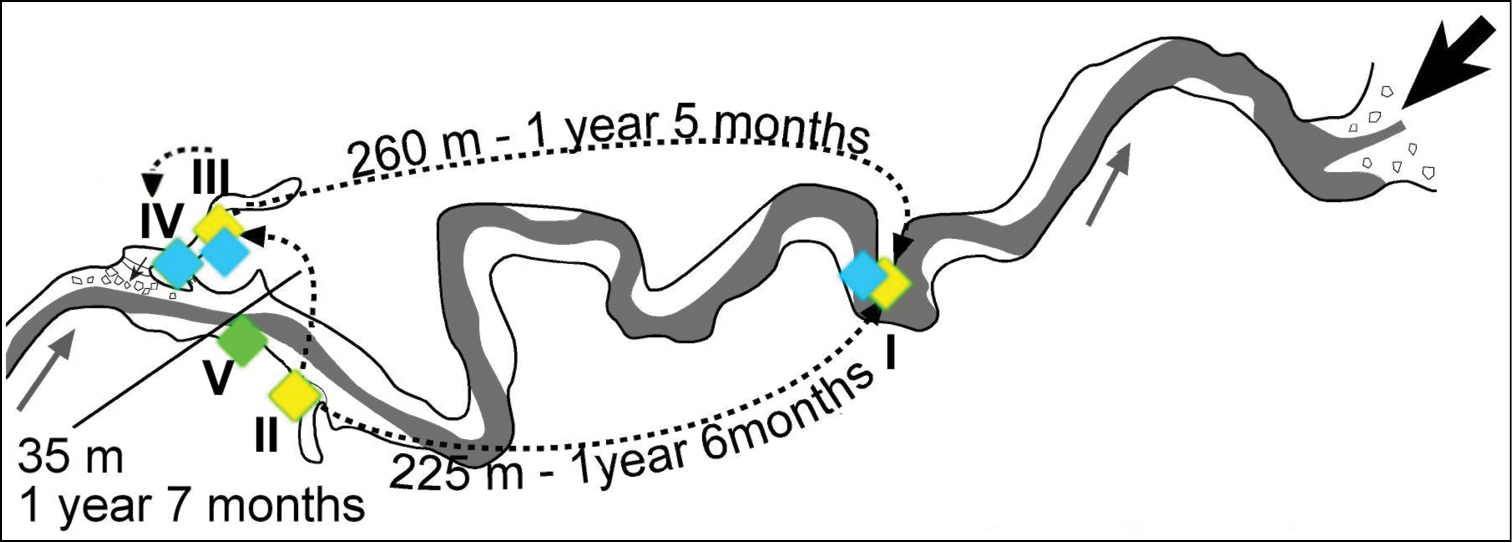
Only 5 marked beetles were found in a different station (Table 1). One yellow marked individual was observed after one and a half year at station I. A blue marked individual was observed one year and five months later at the same station. A third one (yellow marked) was observed at station III after one year and seven months from the marking. Two individuals marked with blue were observed in station IV after 9 months and after almost 2 years, respectively. The covered distances were 225 m (between stations II and I), 260 m (between stations III and I), 35 m (between stations II and III) and 10 m (between stations III and IV), respectively. In their travel, the yellow marked individuals had to cross the stream between stations II and III (Fig. 7).
The migration routes of Drimeotus viehmanni inside Peştera cu Apă din Valea Leşului: yellow = mark for individuals at station II, blue = mark for individuals at station III.
The temperature in the studied cave oscillated from 8 to 9.4°C, higher than during the period 1972–1974 (
The observed number of Drimeotus viehmanni was much higher during our study than during the period 1972–1974. In the previous study,
The fluctuation of the number of individuals presented the same seasonality as in the cases of Pigailh Cave (
The estimated population size presents high variability (between 5, 084 and 147, 764 individuals at station II and between 13, 845 and 533, 033 individuals at station III) inferred from the small number of recaptured beetles. The low resolution of this estimation is similar to that observed for Speonomus and Pholeuon representatives (
The metapopulation concept provides explanations for how species can survive in fragmented landscapes, such as subterranean habitats (
Although dispersal is critical for the population persistence, particularly for small populations (
Data on dispersal of subterranean beetles are rare, despite the theoretical and practical importance for the conservation of these highly endemic taxa. Knowledge of the movement rules can predict the dispersal capacity and patchwise migration rates in complex landscapes (
We thank to Fejér Anna, Milik Csongor and Popa Flaviu for the field assistance. The suggestions of Mauro Rampini and Valerio Sbordoni improved the quality of the manuscript. OTM was supported by the Romanian Academy.