(C) 2013 Marconi Souza Silva. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Lentic cave habitatsare almost always heterotrophic habitats where there are food and oxygen input from the surface. This hydrological exchange seems to be the key factor shaping most groundwater communities. Litter processing in cave water environments has not been experimentally studied as much as it has in lotic subterranean systems, although detritus is likely a critical resource for organisms inhabiting shallow groundwater habitats. The present study sought to evaluate the processing rates and the nitrogen and phosphorous dynamics in plant debris deposited in lentic habitats of two Neotropical limestone caves during 99 days. 84–10×10 cm2 litterbags with mesh sizes of 0.04 mm2 and 9 mm2 were used. In each weighed litter bag, 50 green, intact plant leaf disks (± 2.0 gr/bag) were conditioned. At the end of the experiment, the average weight loss was only 17.4%. No macroinvertebrates were found associated to the debris, but significant differences in the processing rate in relation to the cave and mesh size were observed. The weight loss rate of the plant debris was considered slow (average 0.003 K-day). The amount of nitrogen and remaining phosphorous in the plant debris in the two caves showed variations over time with a tendency to increase probably due to the development of microorganisms which assimilate nitrogen and phosphorus. The slow processing rate of the plant debris can be due mainly to the fact that these lentic cave habitats are restrictive to colonization by shredder invertebrates. Furthermore, the abrasive force of the water, which plays an important role in the processing and availability of fragmented debris for colonization by microorganisms, is absent.
Caves, decomposition, plant debris, nutrients
The groundwater systems are heterotrophic habitats where food and oxygen availability are determined by importation from the surface. This hydrological exchange seems to be the key factor shaping most groundwater communities. Together with oxygen and organic matter, stygoxene and stygophile faunas also have access to the groundwater. If the food and oxygen supply are sufficiently high, these species are able to durably colonize the groundwater and compete with stygobites (
The resource supply in cave ecosystems can vary greatly temporally in the same cave and among caves within a limited geographic area and in some cases can be similar to that in many surface stream types (
However, there is a larger movement of leaves and trunks in the epigean environment as well as in the hypogean environment in periods of intense rain, when the water speed and flow are increased (
The decomposition or processing of debris occurs in successive degradation stages with the consequent release of nutrients such as nitrogen and phosphorous. These stages are influenced by the activity of physical-chemical agents such as current speed, temperature, pH, oxygen, dissolved nutrient content and the present plant or animal tissues, and also by the biological activity such as the meso- and microorganism actions (
Decomposition of leaf litter has been widely investigated in both aquatic and terrestrial environments (
The present study aims to evaluate the processing rates of the coarse particulate organic matter and both nitrogen and phosphorous dynamics in plant debris exposed to decomposition in the lentic habitats in two Neotropical limestone caves.
The study was carried out from April to July of 2009 in the Brega and Santuário limestone caves (Brega - 419447.15E, 7742205.17S, elevation 725 m and Santuário 419312.50E, 7742176.54S, elevation 722 m), located in the municipal district of Pains, Minas Gerais, Brazil. The caves have permanent groundwater and only Brega cave receives also water from rain floods.
The Brega Cave had predominantly vadose genesis, presenting a horizontal development of approximately 760 meters and two opposite entrances. Santuário Cave had also predominantly vadose genesis and presents a horizontal development of approximately 700 meters and two entrances. At the end of the main conduits of the two caves are small perennial water holes feed by upwelling groundwater.
According to the classification of Köppen (
The local tropical climate is responsible for the seasonal variation of the hydric dynamics of the ribeirão dos Patos, significantly altering the surrounding landscapes. The wet season lasts from October to March, which according to
Rates of litter breakdown in lentic environments were measured using bags containing 50 intact plant leaf disks (area = 63.6 mm2/each disk, ± 2 grams). The bags with 0.04 mm2 and 9 mm2 meshes, measuring 10 × 10 cm2 on the sides, enable the microinvertebrates colonization (
The leaves were collected from trees (Moraceae: Ficus sp.) located in the surrounding area before the fall of leaves, thereby reducing the possibility that the experimental material had been previously exposed to decomposition after falling on the ground.
Triplicates of each mesh size were removed (after intervals of 14, 35, 50, 64, 78, 85 and 99 days) to quantify the decomposition through the weight loss and to determine the composition of the associated invertebrate fauna. Before the incubation of the litter bags in the cave water, the initial weight and the initial nitrate and phosphate levels present in the foliar portions were measured. After each collection, the material was washed with distilled water (
The remaining plant material and the wash water were oven-dried at 100° C for 48 hours and later weighed. After the material was dried and weighed, it was sent to the Plant Nutrition Laboratory of the Federal University of Paraná, Brazil, where the analysis of remaining nitrogen and phosphorous in the fractions under decomposition was conducted. Total leaf nitrogen and phosphorus were also measured, using the microKjeldahl method on another portion of the leaf (
To evaluate the influence of the moisture of the green leaves on the weight loss measurements, 50 green leaves were weighed (2.03 gr), oven-dried and reweighed (0.48 gr). Thus, the 76.3% plant disk weight loss is due to the moisture loss and not the mass loss. Water samples were collected from each cave on the sampling start date and analyzed for turbidity, dissolved oxygen, phosphorus and nitrogen.
The amount of organic matter remaining after exposure was expressed in percentage (%) of remaining dry weight [ (FW × 100) /IW]. Where IW is the initial weight of the sample at time zero, FW is the final weight of the sample at time t+1. The decay processing rate (k-day) in each cave was described by the model Mt = M0 e-Kt (
To determine the processing speed (k/day) of plant debris we compared our results to data from the literature for aquatic environments and defined slow (≤ 0.005), moderate (0.006–0.10) and fast (0.10–0.15) processing (Table 1). To evaluate differences in the average final weight values among sites and among the dates of collection and in the same different mesh types, the t Test was used (
Decay process (k-day) in this study compared to other published work from aquatic environments: Standard error (se).<br/>
| Habitat type | k-day | se | References |
|---|---|---|---|
| Brega cave water (mesh size 0.04 mm2) | 0.0025 | 0.0005 | This study |
| Brega cave water (mesh size 9 mm2) | 0.002 | 0.004 | This study |
| Santuário cave water (mesh size 0.04 mm2) | 0.003 | 0.000 | This study |
| Santuário cave water (mesh size 9 mm2) | 0.0032 | 0.000 | This study |
| Cave pool | 0.003–0.001 | - |
|
| Ephemeral river pool | 0.009 | - |
|
| Littoral zone, Lake | 0.0058–0.0039 | - |
|
| Chaney Lake | 0.0025 | - |
|
| Cave stream | 0.0158 | - |
|
| Cave stream | 0.003 | - |
|
| Cave stream | 0.043–0.598 | - |
|
| Cave stream | 0, 03-0.05 | - |
|
| Cave stream | 0.0173 | - |
|
| Cave stream | 0.001–0.012 |
|
|
| Cave stream (mesh size 10×8 mm) | 0.004–0.012 |
|
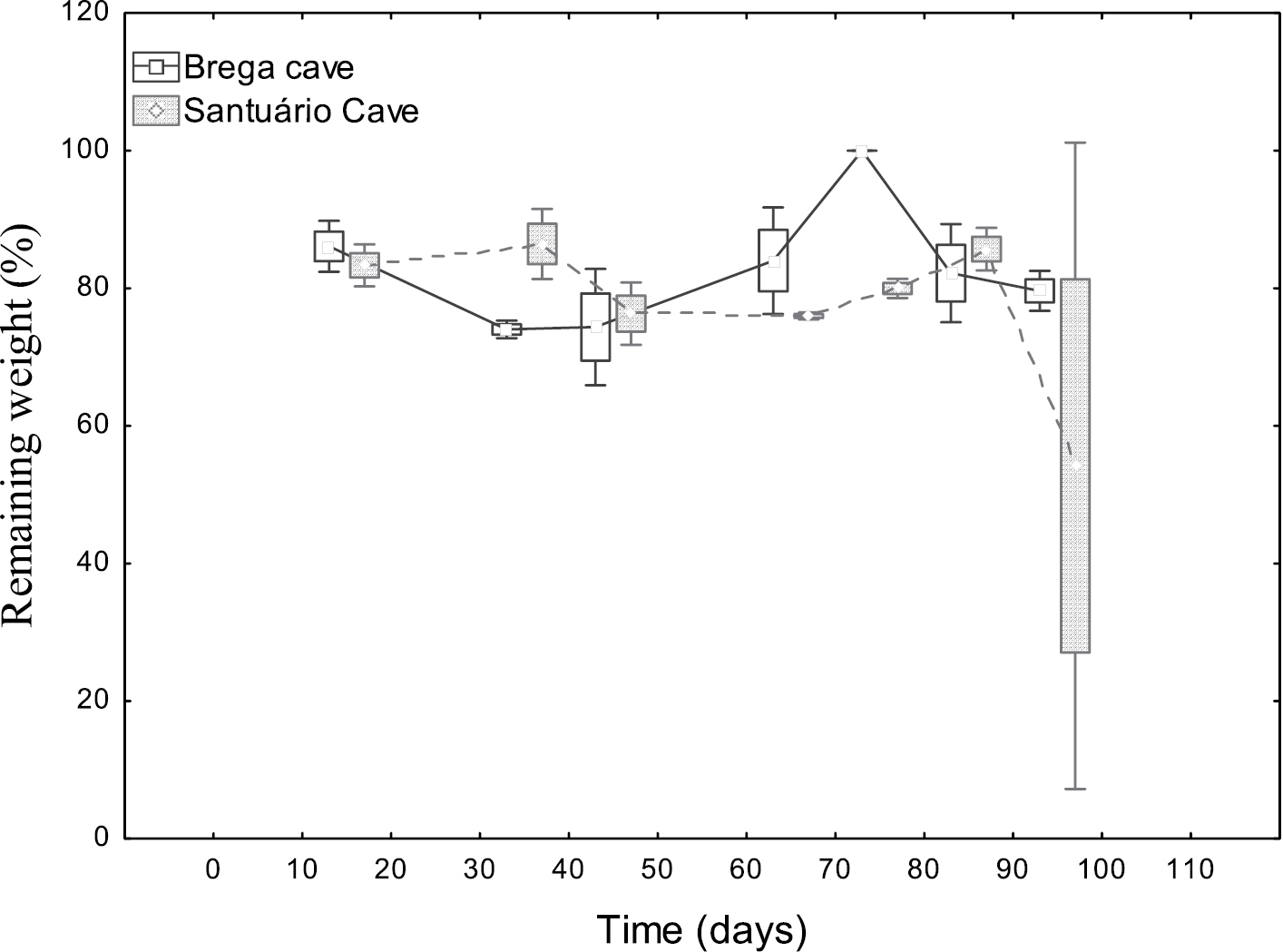
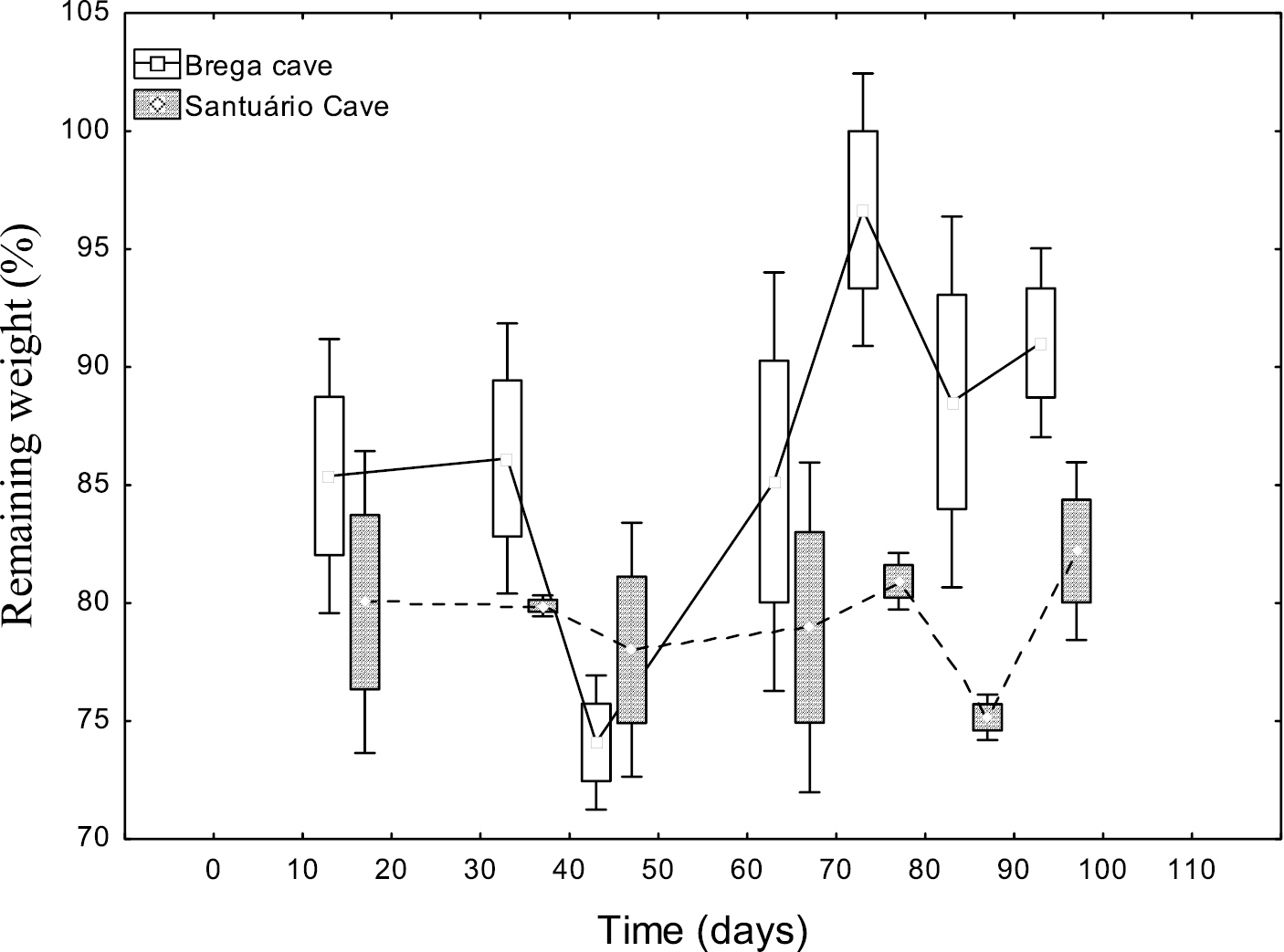
The plant disks exposed to decomposition presented slow processing speed, but varied with time and between the two exposure caves (Table 1, Figs 1 and 2). However, significant differences were observed in the average final weight between both the two caves and the two mesh sizes (Table 2).
In Brega Cave, during the first 14 days of exposure of the 0.04 mm2 mesh an average remaining weight of 86% was observed. After 85 days of exposure the plant debris had an average remaining weight of 82% in relation to the initial mass (Fig. 1). In the same cave, during the first 14 days of the 9 mm2 mesh plant disk exposures, an average remaining weight of 88% was observed. After 85 days of exposure of the plant disks, the average remaining weight corresponded to 88% (Fig. 2).
In Santuário Cave, during the 14 days of exposure in the 0.04 mm2 mesh, an average remaining weight of 83% was observed. After 85 days of exposure of the plant disks, the average remaining weight was 84% (Fig. 1).
In Santuário Cave from the 14th to the 35th days of exposure of the 9 mm2 mesh plant disks, an average remaining weight of 80% was observed. From the 78th to 85th days of exposure the average remaining weight was 78%. At the end of the 99 days of exposure, the average remaining weight corresponded to 82% (Fig. 2).
We can assume that for all of the mesh sizes a slow weight loss rate was registered. As such, we can also consider the low decay (K/day) values (average 0.003 K-day) for the water of these two caves (Table 1).
There were no macroinvertebrates associated with the debris.
Remaining weight of plant disks exposed to processing in the 0.04 mm2 litterbag mesh size in the lentic habitats of Brega and Santuário caves. Mean, Box: Mean±SE, Whisker: Mean±SD
Remaining weight of plant disks exposed to processing in the 9 mm2 litterbag mesh size in the lentic habitats of Brega and Santuário caves. Mean, Box: Mean±SE, Whisker: Mean±SD.
Significant* (p ≤ 0.05) and non-significant differences in the final plant matter weight processing between caves and litterbag mesh sizes in lentic environments: Brega Cave (B), Santuário Cave (S), 0.04 mm2 mesh (1), 9 mm2 (2).<br/>
| Cave | Initial | Mean | Mean | t-value | df | p | Std.Dev. | Std.Dev. |
| mesh | weight | 1 | 2 | Group 1 | Group 2 | |||
| B2 x. S2* | 0.5 | 0.461 | 0.417 | 4.313 | 40 | 0.000 | 0.042 | 0.021 |
| B1 x. B2* | 0.5 | 0.430 | 0.461 | -2.171 | 40 | 0.036 | 0.051 | 0.042 |
| B1 x. S1 | 0.5 | 0.430 | 0.419 | 0.855 | 39 | 0.398 | 0.051 | 0.027 |
| B1 x. S2 | 0.5 | 0.430 | 0.417 | 1.060 | 40 | 0.296 | 0.051 | 0.021 |
| S1 x. B2* | 0.5 | 0.419 | 0.461 | -3.845 | 39 | 0.000 | 0.027 | 0.042 |
| S1 x. S2 | 0.5 | 0.419 | 0.417 | 0.248 | 39 | 0.805 | 0.027 | 0.021 |
The water quality parameters evaluated for the two caves showed quite different values. In Brega Cave the values were as follows: turbidity (6.97 unt), dissolved oxygen (5 mg/l), phosphorus (1.2 mg/l) and nitrogen (0.8 mg/l). In Santuário Cave the values were as follows: turbidity (39.5 unt), dissolved oxygen (4.5 mg/l), phosphorous (1.8 mg/l) and nitrogen (1.4 mg/l).
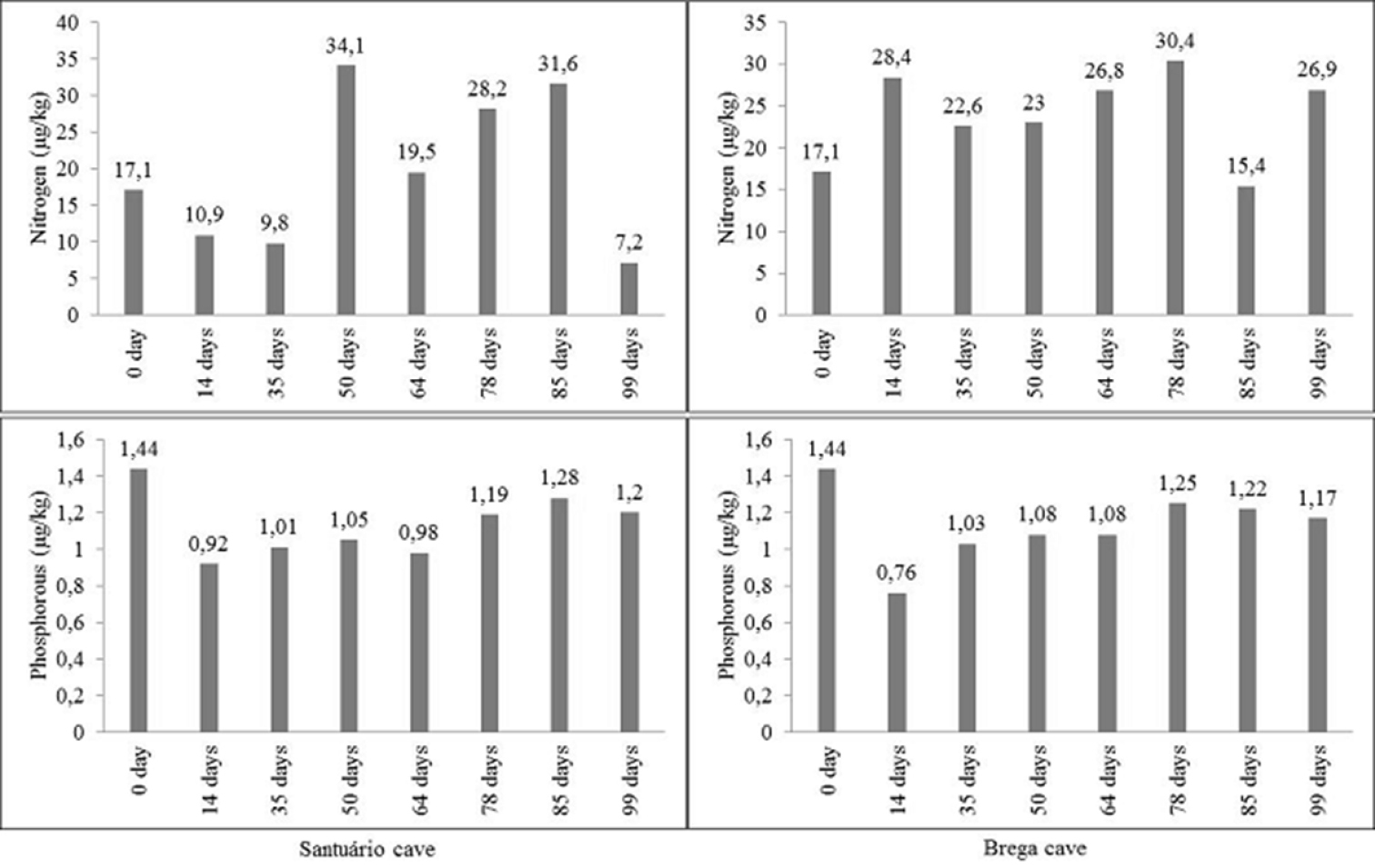
The values for nitrogen and phosphorous in the plant disks in the two caves showed different values during the experiment. However, significant differences were not found in the average amount of nitrogen and phosphorous present in the debris of the litterbags of the two caves.
In Santuário Cave, the initial nitrogen value was 17.1 μg/kg. After 14 days of plant disk exposure (April 18) this value reduced to 10.9 μg/kg. After 99 days of exposure (July 12) the lowest value (7.2 μg/kg) of the remaining nitrogen was registered (Fig. 3). In Brega Cave, the initial nitrogen value was also of 17.1 μg/kg. With 14 days of exposure (April 18), the amount of nitrogen in the plant disks increased (28.4 μg/kg). On the 99th day of exposure of the plant debris (July 12), the nitrogen value was of 26.9 μg/kg (Fig. 3).
In Santuário Cave, the initial value of phosphorous was 1.44 μg/kg. After 14 days of exposure of the plant debris (April 18) the lowest amount of this nutrient was registered, corresponding to 0.76 μg/kg. After 85 days (June 28) there was a small reduction in the amount of phosphorous in the plant debris (Fig. 3). In Brega Cave, the initial phosphorous value was also of 1.44 μg/kg. After 14 days of exposure (April 18) the lowest amount of this nutrient was registered, 0.92 μg/kg. On the 99th of exposure (July 12), the amount of the remaining phosphorous in the plant debris showed a reduction to 1.20 μg/kg (Fig. 3).
Temporal values of nitrogen and phosphorous (μg/kg) measured in plant discs exposed to decomposition in lentic habitats of the Santuário and Brega caves.
Works related to processing of plant debris have not been undertaken in lentic cave habitats until present. Therefore, the data of this study are compared with organic plant matter processing studies conducted in underground and surface streams, ephemeral karst lakes and oligotrophic lakes (Table 1). Some studies revealed that decomposition had different speeds, from slow to fast, in subterranean streams (Table 1).
Organic matter decomposition depends on the action of organisms, physical-chemistry features and abrasive force of the water, all playing an important role in the organic plant matter degradation (
The microorganisms and aquatic shredder invertebrates activity significantly interferes in the organic matter decomposition coefficient, constituting an indirect measure of the use potential of the debris as a food resource (
The slow decomposition rates of the organic plant matter in the lentic environments of Brega and Santuário caves due mainly to the fact that these environments are spatially and trophically restrictive to colonization by shredder invertebrates. Furthermore, the abrasive force of the water, which plays an important role in debris processing and availability, is absent. Thus, lentic caves habitats can be vulnerable to organic matter enrichment when compared to surface aquatic environments or underground streams (
Caves and their microhabitats are often contaminated by nutrients resulting from human activities that are carried by water to the subterranean environment (
In cave aquatic habitats the quantity of organic matter is known as one factor that influences microorganisms and invertebrate fauna structure and physical-chemistry conditions (
The delay in the reduction of the amount of the nitrogen and phosphorous in the plant disks can be due to their low solubility and delay in colonization by microorganisms (
More researches are needed to understand how litter decomposition can be affected both by hydrology and biological communities. The understanding of the debris processing dynamics in the ecosystems and their influence on community structuring is fundamental for the biodiversity conservation in as much these processes represent the energy base of the systems.
The authors are grateful to John Holsinger for English corrections of the manuscript and two anonymous reviewers for their useful suggestions. We express our thanks to the Coordenadoria de Pesquisa do Centro Universitário de Lavras (UNILAVRAS). Leopoldo Ferreira de Oliveira Bernardi, Mr. Brega (proprietário da fazenda), Rosinei (Coelho), Conselho Nacional de Pesquisa (CNPq), Fundação de Amparo a pesquisa do Estado de Minas Gerais (Fapemig), Centro Nacional de pesquisa e conservação de cavernas (CECAV).