(C) 2014 Julie L. Day. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Acanthocephalan parasites infecting the newly described Grotto Sculpin (Cottus specus), a state-threatened and federally endangered troglomorphic fish endemic to Perry County, Missouri, were identified in fish from six cave and four non-cave karst streams. Infection rate and infestation severity were higher among fish from cave streams as compared to non-cave streams. Fish from several caves presented with cases of severe infection and near complete parasite occupation of the intestinal tract. Increased cannibalism and variable water quality are proposed as possible explanations for increased Grotto Sculpin parasitism. This observation suggests that the health of cave fauna may be tied to diet and host population dynamics, and that species subject to severe dietary restrictions and the effects of anthropogenic disturbances may have high vulnerability and conservation risks.
Sculpin, cave, parasite, ecology, acanthocephalan
Comparative studies of host-parasite relationships have shown tremendous potential for illuminating the underlying mechanisms of speciation, dispersal, gene flow, effective population size, and both evolutionary and ecological patterns of occurrence that might otherwise remain obscure (
In cave systems, food scarcity is often cited as a key ecological factor with respect to persistence and resilience for many populations (Eigenmann 1909,
With such variable ecosystem dynamics, the success of parasites of cave fauna is largely dependent on ecology of both the host and parasite. Definitive host specialization, paratenic host requirements, and host biogeography can all affect parasite survival (
Grotto Sculpin (Cottus specus) have recently been distinguished from the Banded Sculpin species complex (Cottus carolinae) and areendemic to cave systems underlying Perry County, Missouri (
In this study, we provide one of the first dedicated investigations of cavefish parasite ecology and explore the efficacy of parasites for comparing habitat-specific infection rates as indicators of the prevalence of fish parasites in cave ecosystems. In Grotto Sculpin, we sought to identify potential at-risk populations and those most susceptible to environmental perturbations from surface ecosystems by comparing relative parasite loads between cave and surface populations of Cottus specus. We hypothesized that decreased parasite loads would be present in cave populations due to limited food web exploitation and resulting difficulties in locating appropriate paratenic hosts.
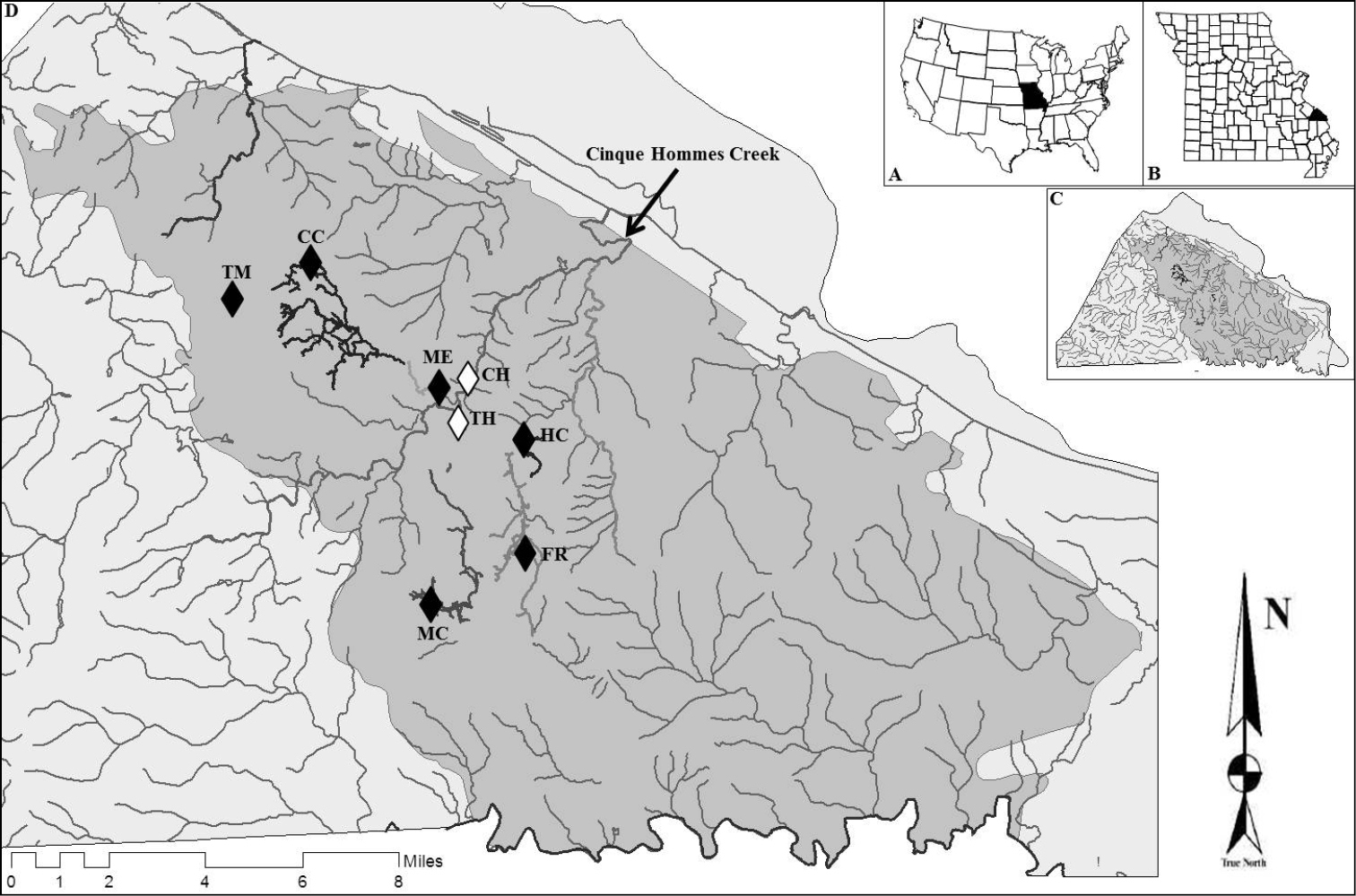
Perry County lies on the Salem Plateau in the northeast Ozark Highlands of southeastern Missouri, USA (Figure 1) and contains 656 known caves, including the states’ four longest (
Panes A–C provide geographic reference within the interior United States. Pane D shows the geographic distribution of grotto sculpin cave systems in Perry County, Missouri and denotes sampling localities in black for caves and white for non-cave streams. Shaded area denotes karst sinkhole plain boundaries, and bolded streams indicate subterranean cave streams. See Table 1 for location abbreviations.
Parasitism by Leptorhynchoides thecatus in Grotto Sculpin from non-cave and cave streams in Perry County, Missouri.
| Site | Abr. | Habitat | N | N Parasitized | N Worms | Prevalence | Intensity | Abundance |
|---|---|---|---|---|---|---|---|---|
| Big Creek | BC | Surface | 29 | 0 | 0 | 0% | 0 | 0 |
| Collier Spring | CS | Surface | 10 | 5 | 7 | 50% | 1 | 1 |
| Cinque Hommes | CH | Surface | 29 | 6 | 10 | 21% | 2 | 0 |
| Thunderhole | TH | Resurg | 18 | 9 | 49 | 50% | 5 | 3 |
| Mertz Cave | ME | Cave | 16 | 9 | 24 | 56% | 3 | 2 |
| Tom Moore Cave | TM | Cave | 32 | 21 | 89 | 66% | 4 | 3 |
| Mystery Cave | MC | Cave | 46 | 33 | 995 | 72% | 30 | 22 |
| Hot Caverns | HC | Cave | 4 | 3 | 115 | 75% | 38 | 29 |
| Crevice Cave | CC | Cave | 11 | 10 | 73 | 91% | 7 | 7 |
| Flaming River | FR | Cave | 34 | 31 | 266 | 91% | 9 | 8 |
| Total | 229 | 127 | 1628 | 55% | 13 | 7 | ||
Two-hundred and twenty-nine Grotto Sculpin were collected using straight line seines 1.8–3.1 m by 1.2 m (3 mm mesh) from selected sampling localities in Perry County, Missouri. Sampling trips were conducted between February 2007 and October 2008. Exhaustive seine hauls were made at two riffle locations per site, and whole specimens were sacrificed in the field by MS-222 overdose. Fish were either preserved in 70% ethanol in the field or frozen whole at -30° in the laboratory. To minimize take from a potentially limited population, the samples we collected were also used in related genetic and morphological analyses aimed at quantifying the status of Grotto Sculpin (
In the laboratory, blunt-tipped scissors were used to carefully cut the ventral body wall from the pelvic girdle to the anus in a manner that would not damage the intestine. The entire digestive tract, from the esophagus to the anus, was removed using forceps from all 229 sculpin. Tracts were flushed with distilled water and examined for intestinal parasites using a binocular dissecting scope at 10–40× magnifications. When present, acanthocephalans were enumerated and suspended in 70% ethanol for fixation and storage at the University of Central Arkansas. Several acanthocephalan specimens with exposed proboscises were isolated from frozen sculpin, cleared, mounted, and morphologically identified as Leptorhynchoides thecatus based on proboscis structure (D. Gettinger, pers. obs.).
Parasitism prevalence was used to descriptively show presence-absence data for Grotto Sculpin populations and allowed us to compare infections rates between our populations (
We examined a total of 229 adult sculpin ranging from 64.5 mm to 76.5 mm SL from six caves, one resurgence, and three surface streams (Table 1). For cave versus surface comparisons, caves were defined as completely subterranean streams requiring penetration to sample, and surface streams, which are only accessible from the surface. For the longest cave sampled, Mystery Cave, samples were collected within the first accessible 3 km of passage. All other caves were sampled haphazardly as fish were encountered. Precise resurgence distances and cave connectivity are lacking, but see
Comparison of sculpin parasitism in samples from cave and surface streams in Perry County, Missouri.
| Habitat | N | N Parasitized | N Worms | Prevalence | Intensity | Abundance |
|---|---|---|---|---|---|---|
| Cave | 143 | 107 | 1562 | 75% | 15 | 11 |
| Non-cave | 86 | 20 | 66 | 23% | 3 | 1 |
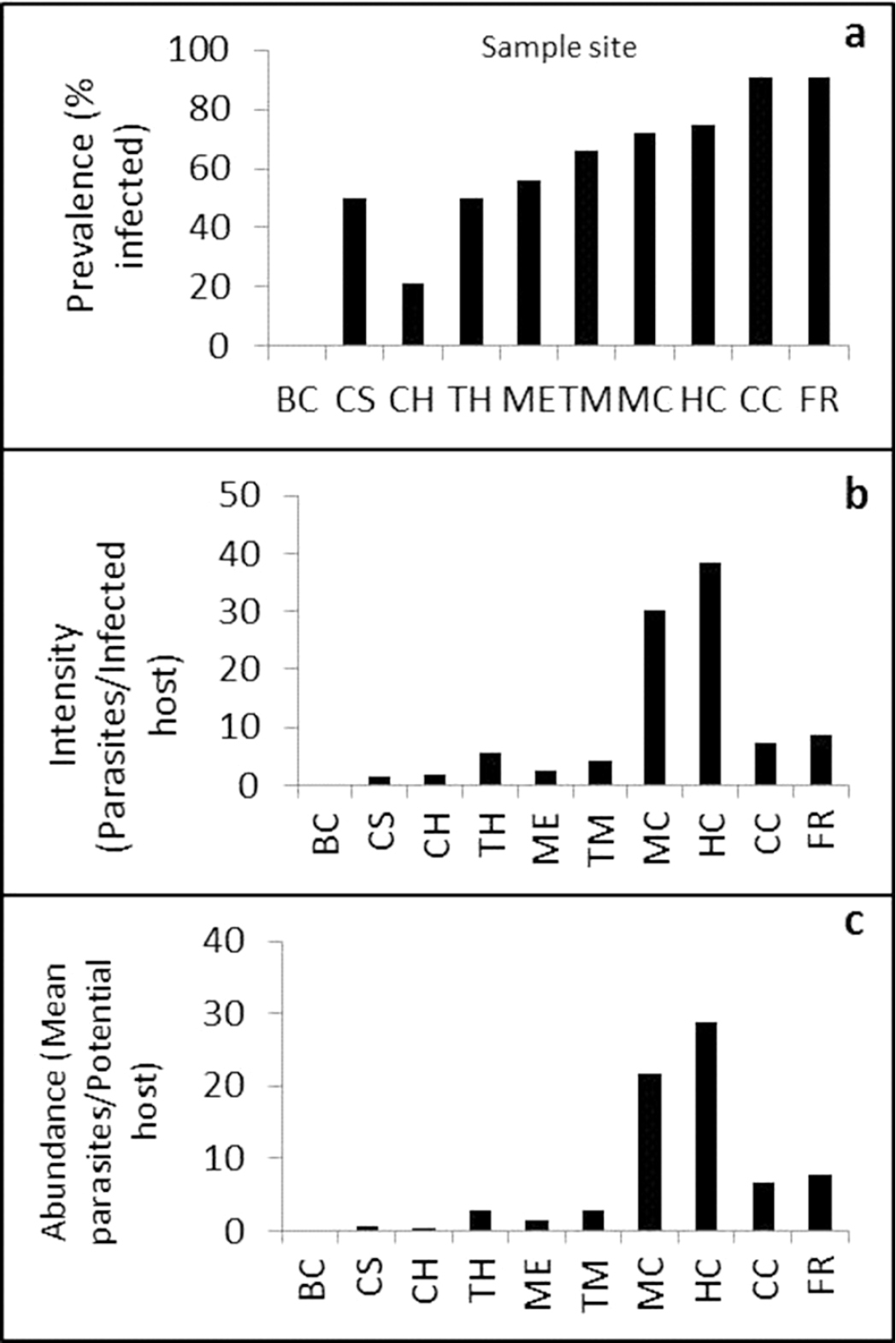
Sculpin were found to be parasitized by Leptorhynchoides thecatus at all sampling sites except one (Big Creek; Table 1). Mean intensity tended to be similar for sculpin at all sites with the exception of Hot Caverns and Mystery Cave where mean intensity rates were much higher than at other sites (Table 1; Figure 2). Mean parasite abundance was significantly different among cave and non-cave locations and showed a similar pattern to mean intensity where all sites were similar except for much higher intensity rates at Hot Caverns and Mystery Cave (p=0.01).
Site specific prevalence (a), intensity (b), and abundance (c) of parasites found in grotto sculpin. Sample site abbreviations correspond with Table 1.
Our observation of what appears to be a successful invasion of the cave environment by acanthocephalans marks the first report of parasitism in a cave-dwelling sculpin and the most inclusive of all cavefish species. Our results revealed that parasitism by Leptorhynchoides thecatus was significantly higher in caves than non-cave stream populations of Grotto Sculpin. While small sample sizes precluded fine-scale resolution of host-parasite population dynamics in individual caves, our data were sufficient to provide an overview of possible explanations for this unique phenomenon that encompass multiple schools of ecological and evolutionary thought. Here we briefly explore the biological relationships of cannibalism and parasitism, complemented by an account of how their ecological effects may be compounded and magnified by the perils of subterranean life within the context of conservation.
Subterranean systems have traditionally been thought of as simple, unchanging ecosystems that persist in near or total isolation from surface resources, but this assumption has been the subject of much scrutiny and reconsideration in recent years as studies of nutrient contributions and trophic ecology increase (
The cave system inhabited by Grotto Sculpin, is remarkably interconnected and influenced by surface activities and supports the notion of increased complexity. Just as organic matter beneficial to troglobites may enter caves relatively quickly in shallow karst regions, so too can potential contaminants from surface land use practices.
Point- and nonpoint-source pollution events pose notable threats to Grotto Sculpin populations and could increase susceptibility to parasitism as a result of reduced health condition. Mystery Cave supports the largest documented population of Grotto Sculpin, with estimates ranging from 262 to 509 individuals (
In these unique karst systems, extensive movement of groundwater serves not only as a means of nutrient transport and fish translocation, but also as means of increasing parasite dispersal and both nutrient and pollution transport. Mertz Cave, which had the lowest incidence of parasitism for a cave, has the largest and most accessible inflowing entrance relative to the other caves tested, which may allow for a higher influx of nutrients from the surface. This may result in less cannibalism and therefore less parasitism (Table 1). In remote or isolated regions of this cave system, a lower influx of surface energy could offer a narrow range of possible hosts for parasites. Therefore, Leptorhynchoides thecatus could be more likely to parasitize Grotto Sculpin in stream reaches located in far proximity to allochthonous organic input. Coupled with cannibalism, this may also contribute to the elevated rates of parasitism we observed.
Cannibalism is theorized to promote parasitism (
In addition to providing insight on the ecology of Grotto Sculpin and their acanthocephalan parasites, the results of this work elucidate novel data gaps on parasite ecology and their persistence in cave fauna. The results of this study suggest that researchers and managers should take care to investigate the potential influences of parasitism and variation in surface contributions to cave biodiversity population dynamics. Directed studies of the prevalence and epidemiological implications of fish health and parasite infections in cave species are still limited in scope and in need of further inquiry. Future research should seek to understand the population dynamics of parasites in subterranean systems and their impacts on ecosystem ecology and function.
Support for this project was provided by Sigma Xi grant to JL Day at University of Central Arkansas, Missouri Department of Conservation, and U.S. Fish and Wildlife Service. The diligence and dedication of B Pobst of MDC made this project both possible and successful. We are grateful to the Perry County MDC office for use of their facilities, numerous landowners who allowed access to sites, and P Moss of Ozark Underground Laboratory for his unending guidance. We thank G Adams, C Johnson, S Vestal, and K McCabe of UCA. We are immensely grateful to the reviewer of this manuscript for constructive and invaluable input.