(C) 2014 Florian Malard. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Guy Magniez was born on 23 August 1935 at Marey-sur-Tille, a small village in Côte-d’Or (France). He followed high school studies in Dijon and obtained his high school diploma
in 1953. He was an elementary school teacher during one year before
joining the University of Dijon where he passed a Bachelor’s degree in
Natural Sciences in 1958. In 1959, he involved in research at the
Laboratory of Geology and obtained a Master degree by submitting a
research report on the microfacies of crinoidal Bajocian limestones.
Once he successfully passed the Aggregation for secondary education in
Natural Sciences in 1960, he integrated the Research Laboratory of
Animal and General Biology at the University of Dijon under the
direction of Professor Husson. He began with the organization of
practical classes for first-year students and a few years later he
became responsible for organizing practical classes in general biology
and genetics for bachelors. At this time, Guy was in charge of breeding
fruit flies for teaching purpose in addition to breeding stenasellids
for his research activity (see below). Then, he was offered a teaching
assistantship and took the lead of the bachelor program in Natural
Sciences. After submitting his state doctoral Thesis in 1976, he
delivered lectures to under-graduate and graduate students. He became an
associate professor in 1985 and was responsible for preparing graduate
students to become teachers. Guy was an exceptional pedagogue: he
supervised numerous master students (including the first author of this
memorial) and led many students to go into teaching natural sciences. In
addition to his heavy teaching duties, Guy also invested much time
into social and administrative tasks at the University of Dijon. As a
committed educator, Guy was both extremely modest and discrete: he has
always been greatly appreciated by his colleagues and students. He was
appointed knight of the Order of Academic Palms as soon as 1979, and
officer in 1986. His research began in 1960 and he was retired in 1999,
but he was still contributing to national and European research
projects in the 2010’s (
Photography of Guy Magniez.
Most of the information on the life cycle of Stenasellus virei Dolfus, 1897 (Stenasellidae)
are from dedicated studies conducted by Guy during the sixties and
seventies. From 1960 to 1976, he collected several populations of Stenasellus
in the Pyrenees and Cantabria and reared them in the Moulis Cave
(Pyrenees), Antheuil Cave (Côte-d’Or) and in thermostatic rooms at the
University of Dijon. Rearing was a necessary step to document the life
cycle of Stenasellus because the body size distribution of cave populations was truncated, with almost no juveniles due to a strong cannibalism (
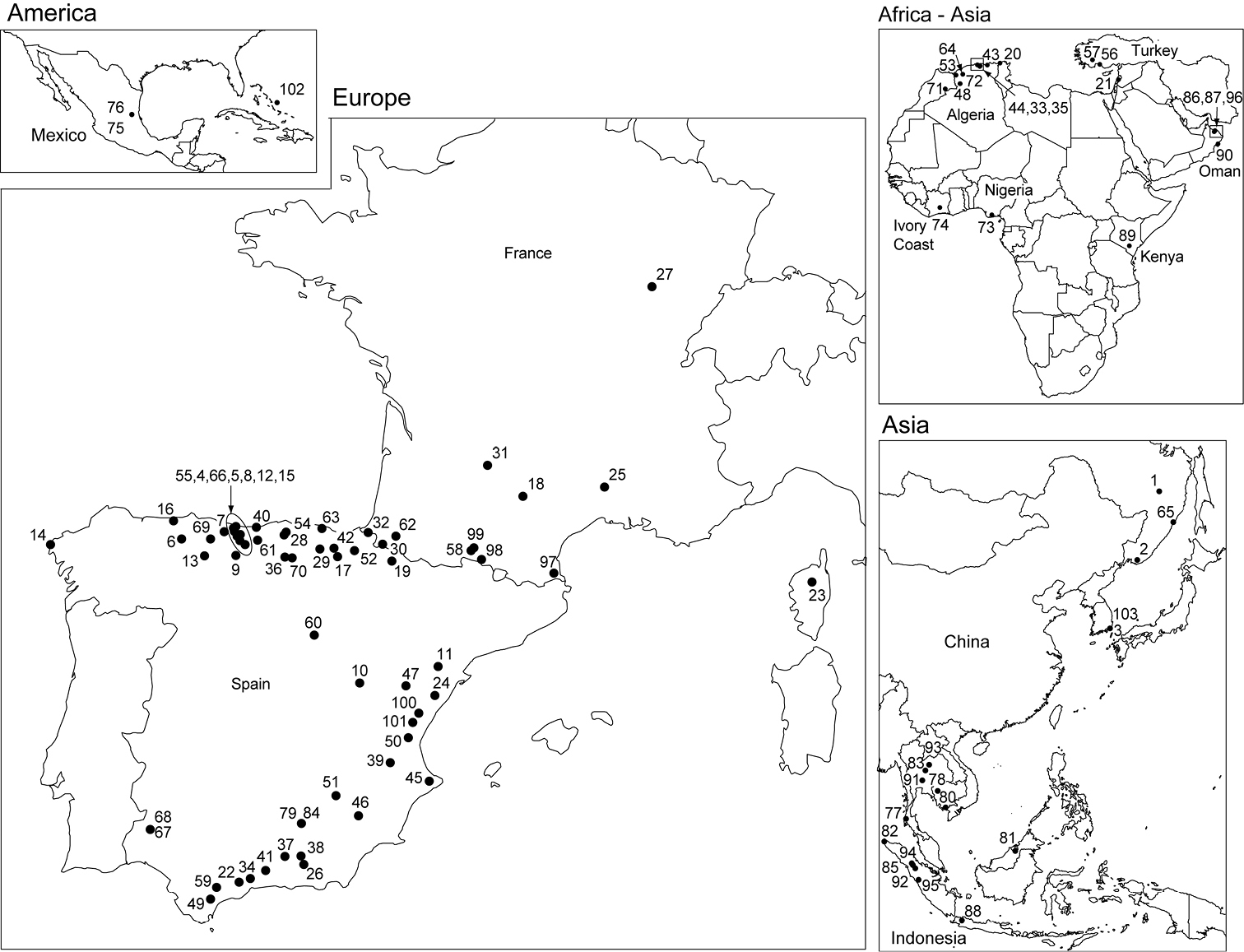
Perhaps, the greatest contribution of Guy consisted in clarifying the systematics of Stenasellidae and Asellidae. He described or co-described a total of 109 taxa among which 6 genera (Bragasellus, Gallasellus, Metastenasellus, Neostenetroides, Parastenasellus, and Sibirasellus), 3 species of Asellus, 13 species of Bragasellus, 48 species of Proasellus, 5 species of Synasellus and 31 species and subspecies of Stenasellidae (Table 1). As his taxonomic knowledge of the Aselloidea
and meticulous morphological descriptions were rapidly recognized by
the scientific community, Guy received biological material from all
over the world and described species in Africa, America, Asia and
Europe (Figure 1). Although Guy never published a single cladogram, his approach of the systematics of Aselloidea
was all about finding the relationships among species through time.
Relationship between taxa was essentially inferred from the shape of
male copulatory organs (second pleopods), the detailed structure of
which was revealed by means of scanning electron microscopy as early as the seventies (
List of genera, species and subspecies described or co-described by Guy Magniez. Numbers refer to the location of species and subspecies as indicated in Figure 1.
| Asellidae |
| 1. Asellus (Asellus) levanidovorum Henry & Magniez, 1995 |
| 2. Asellus (Asellus) primoryensis Henry & Magniez, 1993 |
| 3. Asellus (Phreatoasellus) joianus Henry & Magniez, 1991 |
| Bragasellus Henry & Magniez, 1968 |
| 4. Bragasellus afonsoae Henry & Magniez, 1988 |
| 5. Bragasellus aireyi Henry & Magniez, 1980 |
| 6. Bragasellus bragai Henry & Magniez, 1988 |
| 7. Bragasellus comasi Henry & Magniez, 1976 |
| 8. Bragasellus comasioides Magniez & Brehier, 2004 |
| 9. Bragasellus escolai Henry & Magniez, 1978 |
| 10. Bragasellus lagari Henry & Magniez, 1973 |
| 11. Bragasellus lagarioides Henry & Magniez, 1996 |
| 12. Bragasellus meijersae Henry & Magniez, 1988 |
| 13. Bragasellus molinai Henry & Magniez, 1988 |
| 14. Bragasellus notenboomi Henry & Magniez, 1988 |
| 15. Bragasellus rouchi Henry & Magniez, 1988 |
| 16. Bragasellus stocki Henry & Magniez, 1988 |
| Gallasellus Henry & Magniez, 1981 |
| 17. Proasellus alavensis Henry & Magniez, 2003 |
| 18. Proasellus albigensis (Magniez, 1965) |
| 19. Proasellus aragonensis Henry & Magniez, 1992 |
| 20. Proasellus bagradicus Henry & Magniez, 1972 |
| 21. Proasellus bardaunii Alouf, Henry & Magniez, 1982 |
| 22. Proasellus bellesi Henry & Magniez, 1982 |
| 23. Proasellus beroni Henry & Magniez, 1968 |
| 24. Proasellus beticus Henry & Magniez, 1992 |
| 25. Proasellus boui Henry & Magniez, 1969 |
| 26. Proasellus bouianus (Henry & Magniez, 1974) |
| 27. Proasellus burgundus Henry & Magniez, 1969 |
| 28. Proasellus cantabricus Henry & Magniez, 1968 |
| 29. Proasellus chappuisi Henry & Magniez, 1968 |
| 30. Proasellus chauvini Henry & Magniez, 1978 |
| 31. Proasellus claudei Henry & Magniez, 1996 |
| 67. Synasellus hurki Henry & Magniez, 1995 |
| 68. Synasellus leysi Henry & Magniez, 1995 |
| 69. Synasellus meijersae Henry & Magniez, 1987 |
| 70. Synasellus notenboomi Henry & Magniez, 1987 |
| Stenasellidae |
| 71. Magniezia gardei Magniez, 1978 |
| Metastenasellus Magniez, 1966 |
| 72. Metastenasellus leysi Magniez, 1986 |
| 73. Metastenasellus powelli Magniez, 1979 |
| 74. Metastenasellus tarrissei Magniez, 1979 |
| 75. Mexistenasellus parzefalli Magniez, 1972 |
| 76. Mexistenasellus wilkensi Magniez, 1972 |
| Parastenasellus Magniez, 1966 |
| 77. Stenasellus bedosae Magniez, 1991 |
| 78. Stenasellus boutini Magniez, 1991 |
| 79. Stenasellus bragai Magniez, 1976 |
| 80. Stenasellus cambodianus Boutin & Magniez, 1985 |
| 81. Stenasellus chapmani Magniez, 1982 |
| 82. Stenasellus covillae Magniez, 1987 |
| 83. Stenasellus deharvengi Magniez, 1991 |
| 84. Stenasellus escolai Magniez, 1977 |
| 85. Stenasellus foresti Magniez, 2002 |
| 86. Stenasellus grafi Magniez & Stock, 2000 |
| 87. Stenasellus henryi Magniez & Stock, 2000 |
| 88. Stenasellus javanicus Magniez & Rahmadi, 2006 |
| 89. Stenasellus kenyensis Magniez, 1975 |
| 90. Stenasellus messanai Magnez & Stock, 2000 |
| 91. Stenasellus mongnatei Magniez & Panitvong, 2005 |
| 92. Stenasellus monodi Magniez, 2000 |
| 93. Stenasellus rigali Magniez, 1991 |
| 94. Stenasellus stocki Magniez, 2001 |
| 95. Stenasellus strinatii Magniez, 1991 |
| 96. Stenasellus vermeuleni Magniez & Stock, 2000 |
| 97. Stenasellus virei angelieri Magniez, 1968 |
| 98. Stenasellus virei boui Magniez, 1968 |
| 99. Stenasellus virei hussoni Magniez, 1968 |
| 100. Stenasellus virei margalefi Magniez, 1996 |
| 101. Stenasellus virei rouchi Magniez, 1996 |
| Gnathostenetroidae |
| Neostenetroides Carpenter & Magniez, 1982 |
| 102. Neostenetroides stocki Carpenter & Magniez, 1982 |
| Janiridae |
| 103. Mackinia birsteini Henry & Magniez, 1991 |
Maps showing the location of the 103 species and subspecies of aquatic isopods described or co-described by Guy Magniez. Numbers indicate taxa, the names of which are provided in Table 1.
Following the seminal work of
In his last poster presented at the 19th International Symposium of Subterranean Biology in Fremantle, Australia (21-26 September 2008),
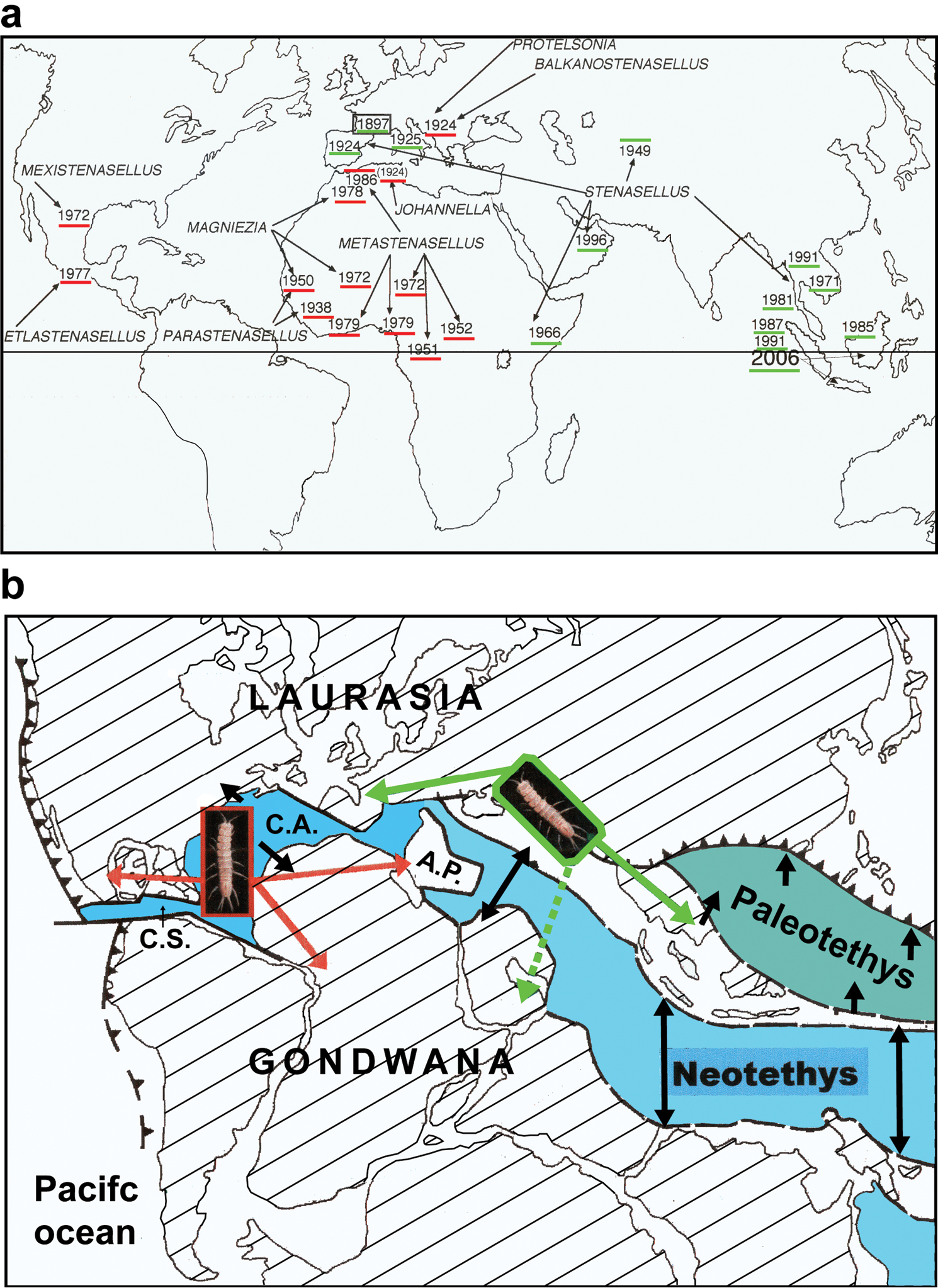
a. Chronology of the discovery of
stenasellids. Red and green lines refer to as the “Atlantic” and
“Mesogean” stocks as indicated in the lower panel. The genus Acanthastenasellus Chelazzi & Messana 1985 (Somalia) was not mapped by Guy b
Hypothetic colonization of groundwater by ancestor of the stenasellids.
C.S. Caribbean sea C.A. Central Atlantic Ocean A.P. Apulian plate.
Panels a & b are modified after
Guy has voluntary remained unprecise as to when the
colonization of groundwater by marine ancestors and diversification of
groundwater lineages occur. This is because he has always been much
skeptical about the assumptions of the climatic relict hypothesis. In
particular, he never associated inland groundwater colonization to any
marine transgression because colonization was necessarily an active
process to him. Moreover, he was convinced that some species of
asellids and stenasellids had experienced considerable range expansion
by dispersing into extensive alluvial systems (
To end up this memorial, we provide below two biogeographic scenarios elaborated by Guy that would warrant further testing by today’s generation of subterranean phylogeographers. The first scenario is an attempt to explain the distribution of stenasellids at global scale (Figure 2). It was a part of his poster presented at the 19th International Symposium of Subterranean Biology but Guy did not publish this work. Guy hypothesized that the ancestors of stenasellids were anophtalmous thermophilic burrowers living in coastal unconsolidated sediments of the Neo-Tethys Sea, the sediments of which accumulated from Trias to Eocene. He further suggested on the basis of morphological taxonomy that the initial colonization of groundwater gave rise to two distinct groups of stenasellids. The first one, to which he referred as the “Atlantic stock”, includes species from the New World, West Africa and the Balkans (red lines in Figure 2). He speculated that the drift of the Apulian block (a fragment of Gondwana) and its fusion with Eurasia might account for the presence of Protelsonia Méhely and Balkanostenasellus Cvetkov in the Balkans. The second group, to which he referred as the “Mesogean stock” corresponds to the genus Stenasellus Dolfus which extends from southern Europe (Iberian Peninsula, southern France, and Italy) to the eastern horn of Africa and Asia. According to Guy’ predictions , a phylogenetic tree of Stenasellidae would exhibit a clear basal dichotomy with all genera except Stenasellus clustering into a monophyletic group and all Stenasellus species clustering into another monophyletic group.
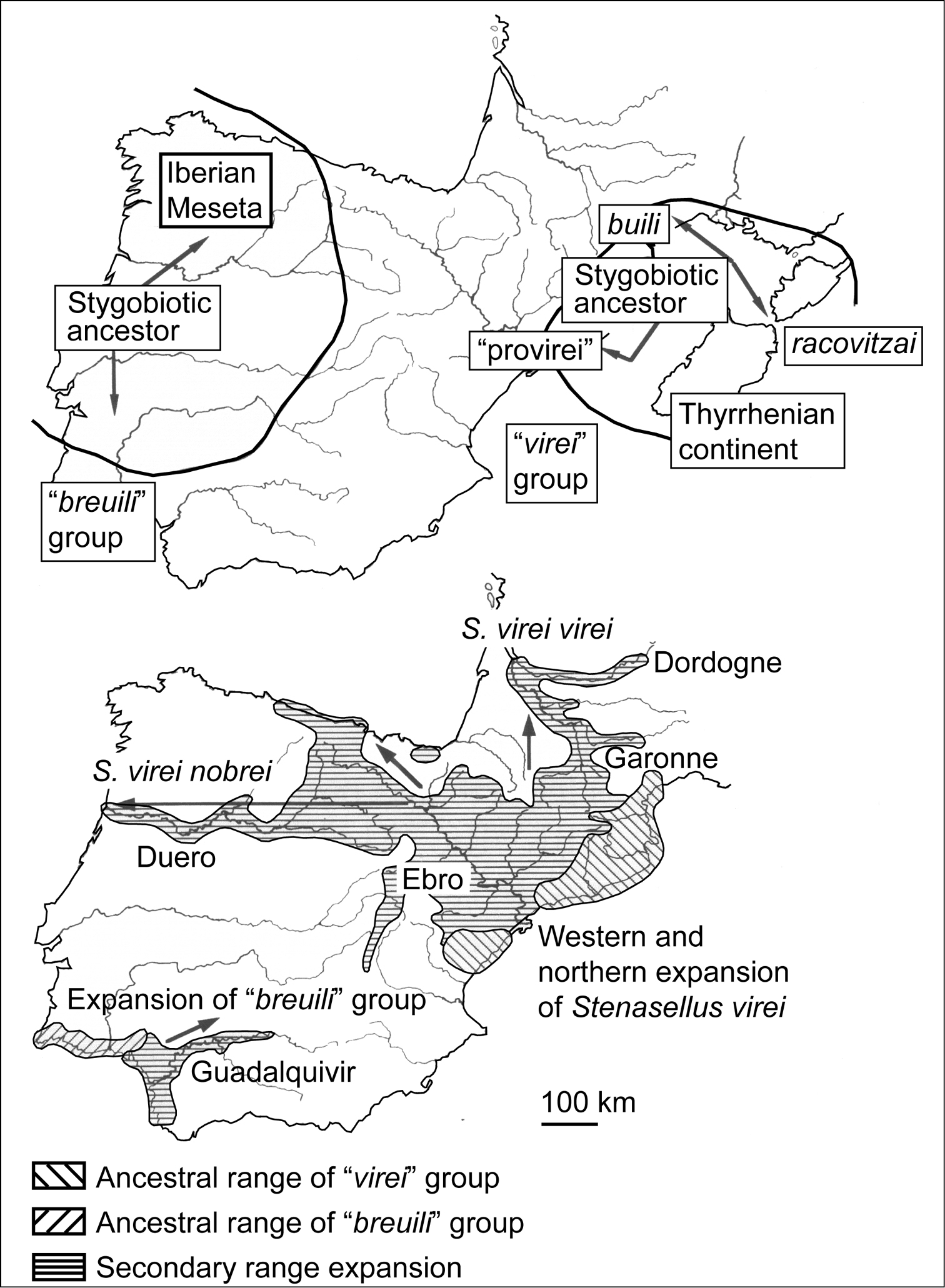
The second scenario attempts to explain the evolutionary history of the Iberian stenasellids (
Paleobiogeography of the Ibero-Aquitanian
stenasellids. Upper panel. Palaeogeographic continental areas attributed
to the stygobiotic ancestors of the “breuili” and “virei”
groups before migration of the Corsica-Sardinia plate. Limits are very
approximate. Lower panel. Recent expansion of geographic ranges of the “breuili” and “virei” groups due to dispersal along large Ibero-Aquitanian river systems. Modified after
Guy Magniez devoted much of his life to the taxonomy of the Aselloidea and described, named and classified more than 100 taxa. Yet, his scientific contribution goes well beyond the taxonomy of the Aselloidea. All along his career, he attempted to merge information from biology, ecology, and evolutionary biology to explain the geographic distribution of groundwater species through geological time. His name would be forever associated to the modern systematics of the Aselloidea but he has also left us many predictive biogeographic scenarios that warrant further testing.
Publications by Guy Magniez
Authors: Florian Malard, Jean-Paul Henry, Christophe J. Douady
Data type: References list.
Explanation note: A list of 153 articles authored or co-authored by Guy Magniez.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/ ). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Link: doi: 10.3897/subtbiol.13.7412.app1