Citation: Di Russo C, Rampini M, Latella L, Cobolli M (2014) Measurements of the diet in two species of Troglophilus Krauss, 1879 cave crickets from Italian subterranean habitats (Orthoptera, Rhaphidophoridae). Subterranean Biology 13: 45–54. doi: 10.3897/subtbiol.13.6719
The diet of two populations of cave crickets, Troglophilus cavicola from Veneto, northern Italy and Troglophilus andreinii from Apulia, southern Italy, were studied by analyzing faecal and gut contents. The results obtained document different food preferences in these two species. In the Troglophilus cavicola population arthropod remains were dominant in the diet, whereas in the Troglophilus andreinii population vegetables (green and fibres) were the more abundant food category. Furthermore, study of the overlap of food resource exploitation among age and sex sub-samples seems to indicate a separation of diet among the young instars and other age classes of the populations. Differences in diet between males and females were observed only in the population of Troglophilus andreinii.
Troglophilus, Troglophilinae, Rhaphidophoridae, trophic niche, cave crickets, feeding habits, Italy
In general, the feeding habits of Rhaphidophoridae cave crickets are considered to be omnivorous–saprophagous (
An example of predatory habits was reported by
In a more recent study, conducted on some Italian populations of the genus Dolichopoda, the trophic niche has been quantitatively described (
The present study is part of a more extensive research project investigating the population ecology of the Italian species of Troglophilus cave crickets (
The genus Troglophilus has a wide east-mediterranean distribution, with 15 species occurring from Austria and Italian pre-Alps to the Balkan-Anatolian region (
This study is based on the analysis of 225 faecal and gut content samples collected periodically from August 2004 to October 2005. In Troglophilus cavicola we analysed 76 samples (56 feculae and 20 guts) from Covoli di Velo cave in the Lessini Mountains (Grotta Inferiore dei covoli di Velo 44 V/VR, altitude 878 m a.s.l., Velo Veronese, Verona). For Troglophilus andreinii, an endemic species of Apulia, we analysed 149 samples (98 faeces and 51 guts) from Tranese cave [Grotta della Masseria Tranese 486 Pu/BA, altitude 330 m a.s.l., Contrada Tranese, Putignano, Bari]. Sub-samples from individuals of different sex and developmental stages (adult, nymphs and young) were analyzed. In particular for Troglophilus cavicola, we utilized 24 adults, 26 nymphs and 26 young instars, while for Troglophilus andreinii 52 adults, 47 nymphs and 50 young instars have been used. The distinction in these three age classes is based on the maturation of sexual characters for the adults and the hind tibia lenght for the other two classes (Nymph = hind tibia included between 15 and 21 mm; Young instar = hind tibia < 15 mm). Fresh faeces were collected from individuals put in a plastic box separately for 24 hours without any food supply. Gut samples were obtained by dissection of fresh individuals. Both faecal and gut pellets were conserved in alchool 70%. The comparison among gut and faecal samples does not reveal any differences in their content, permitting us to pool data obtained by these two types of source.
Feacal and gut contents were spread in Euparal on 24×24 mm slides and examined with an optical microscope (Leica M.Z.12.5). The material analysed was classified into three categories: green vegetables, vegetable fibres and arthropod remains. In order to obtain quantitative estimates, we used a 576 mm2 grid and the food items scored were recorded as proportion of the total area observed. The niche breadth was cumulatively evaluated, for each periodical sub-sample, by the Gini-Simpson index (
B = 1- ∑pi2
where pi = the proportion of the ith item in the faecal or in the gut content.
The overlap in the resource exploitation among age and sex sub-samples was investigated by the
Mo = 2 ∑ pij pik / ∑ p2ij +∑p2ik
where pij and pik are the proportions of ith item utilization by the jth and kth populations.
The examined specimens and slides are deposited in the collections of the Museo Civico di Storia Naturale of Verona and of the Dipartimento di Biologia e Biotecnologie “Charles Darwin”, Università degli Studi di Roma “Sapienza.
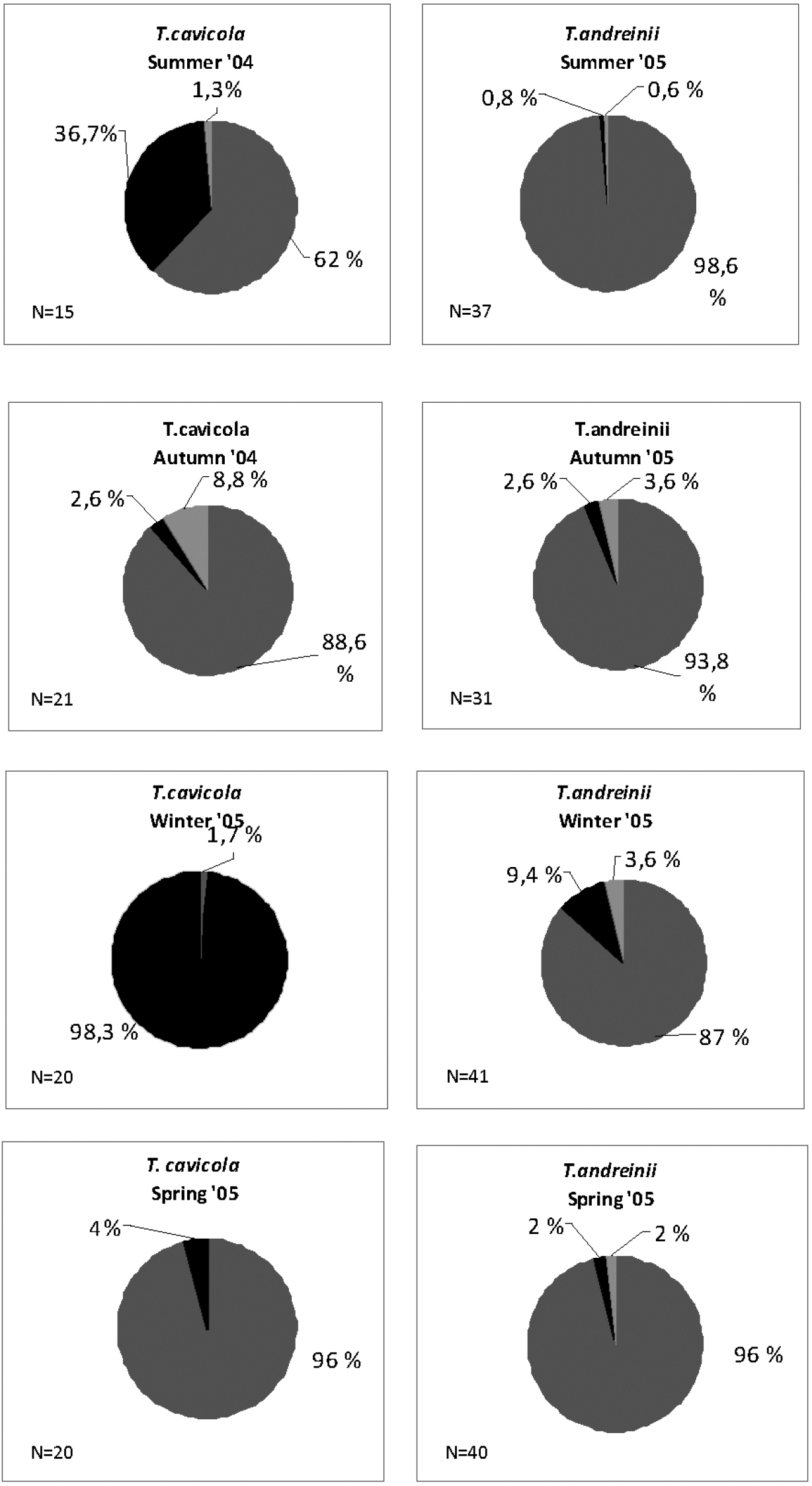
The percentages of utilization of the food resources are compared in the two species over the entire year. For the Troglophilus cavicola population (Covoli di Velo), we observed the dominance of arthropod remains in the diet (69.44%); by contrast, in the Troglophilus andreinii population (Tranese cave), vegetables (green and fibres) were the more abundant food category, reaching about 92%.
Fig. 1 reports comparisons among periodical samples of the two populations. In this case, differences in the exploitation of food resources in different periods of the year are evident. Particularly in the Troglophilus cavicola population, there is a clear increase of vegetable fibres in summer samples and a complete shift to the exploitation of arthropods remains (over 98%) in winter. By contrast, in the Troglophilus andreinii population, the diet is mainly based throughout the year of vegetables (never below 80%), with a small increase of fibres and arthropods in autumn and winter.
Seasonal comparison of food resource exploitation among cumulate samples of Troglophilus cavicola and Troglophilus andreinii. Grey: green vegetables; light grey: fibres; black: arthropod remains.
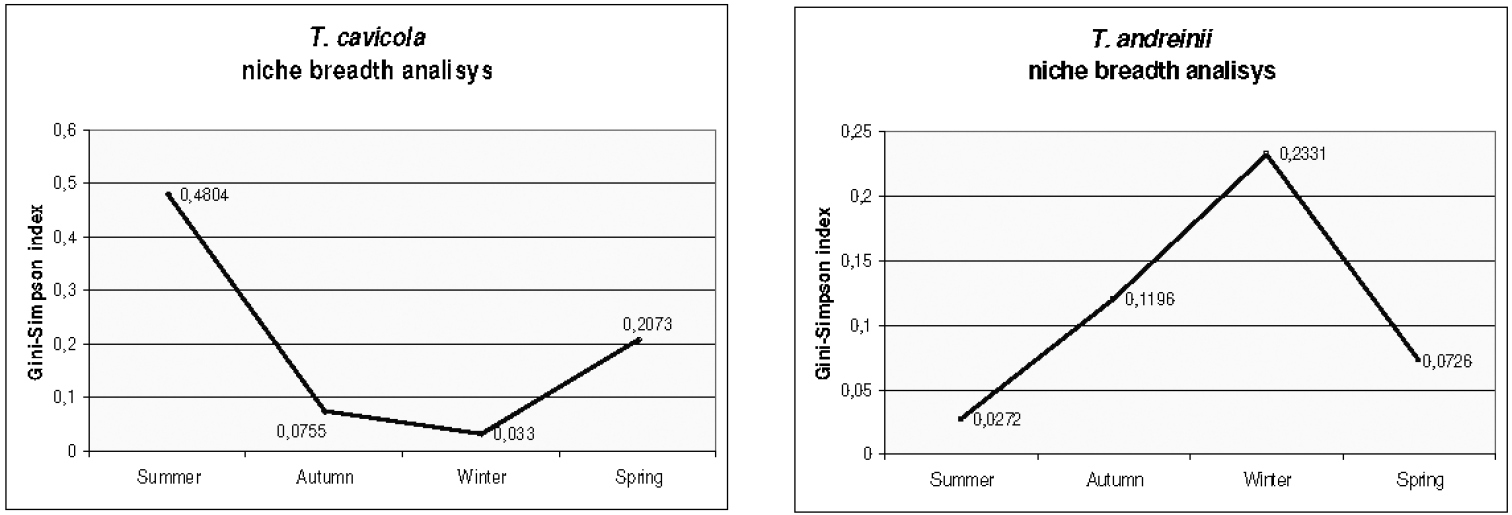
These findings are confirmed by the trophic niche breadth analysis conducted on the periodical samples of the two populations. In Fig. 2 seasonal values of the Gini- Simpson index for the two population are compared, showing an opposite trend of variation. In particular, in Troglophilus cavicola, which has a mean value of the index of 0.44, the highest values of the niche breadth are found in summer and spring, where all the three food categories were exploited. The lowest values occur in winter, where mainly arthropod remains were used. By contrast, in Troglophilus andreinii the mean values of the Gini-Simpson index were significantly lower (0.16), with a moderate peak in winter corresponding to a more balanced exploitation of the three resources.
Comparison of seasonal niche breadth in Troglophilus cavicola and Troglophilus andreinii populations
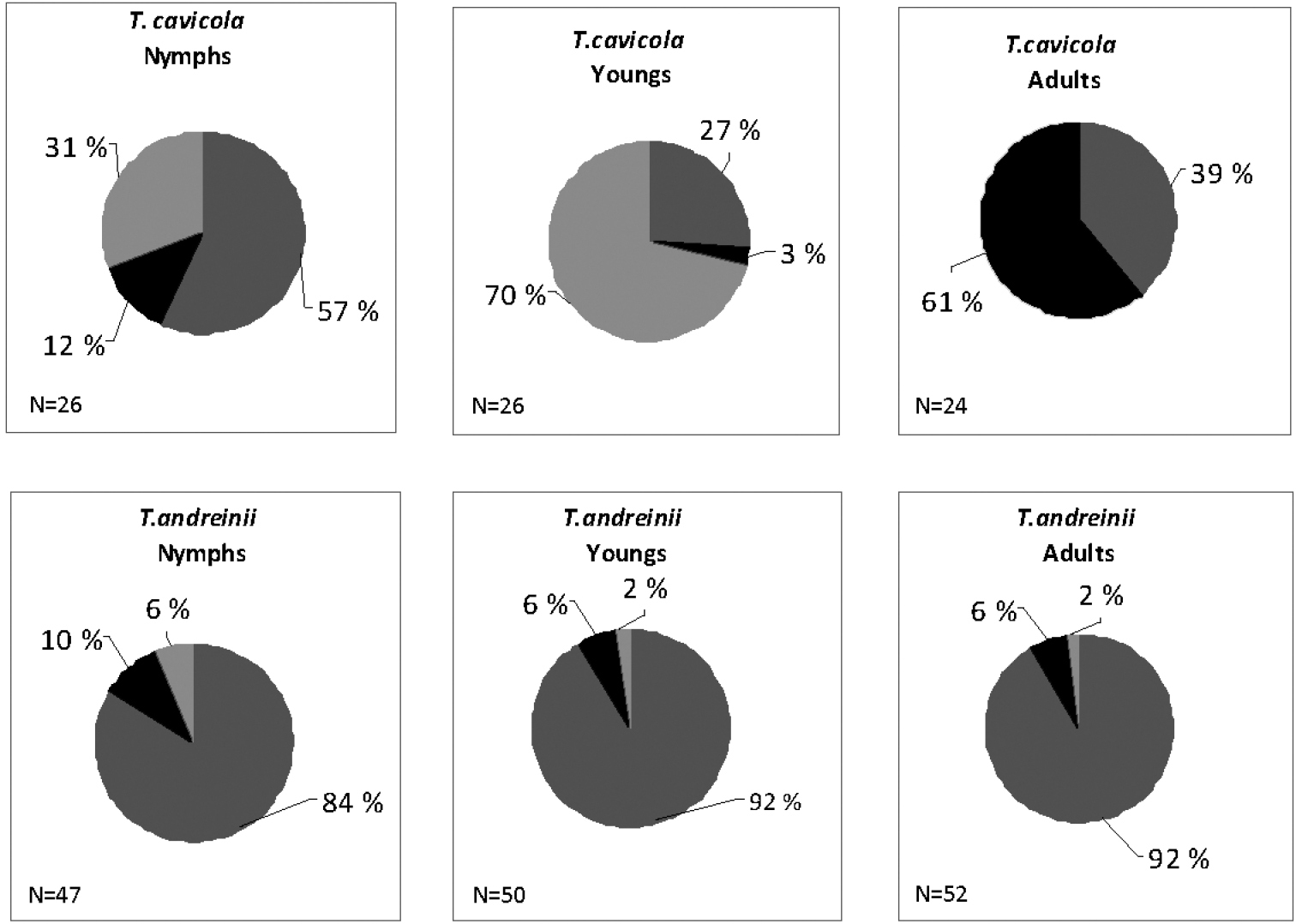
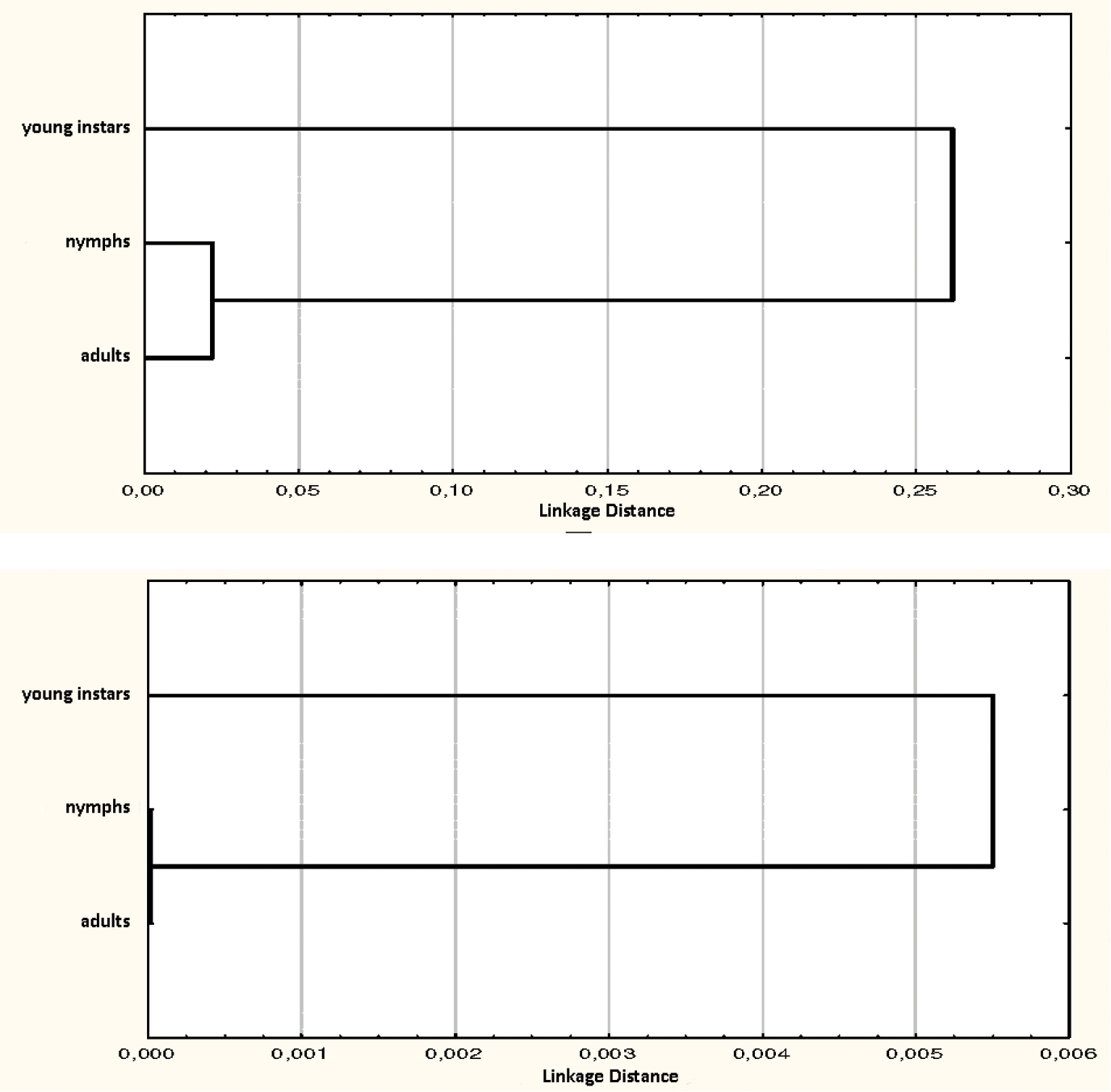
Fig. 3 compares the annual diet of different age sub-samples. In both populations young individuals show a significantly different exploitation of resources in comparison to those of nymphs and adults. In particular, these differences are greater in the Troglophilus cavicola population than in that of Troglophilus andreinii. The diet of young instars of Troglophilus cavicola is mainly based on vegetables (fibres+vegetable matter = 69%), whereas arthropods are dominant in nymphs and adults. In the Troglophilus andreinii population the diet of the three age classes appears more balanced, with a little difference in the young instars where a moderate increase of arthropods occurs. These results can be described by the overlap analysis using the Morisita index (Fig. 4). The dendrograms performed using Euclidean distances based on matrices of this index clearly show a separation of the diet of the young instars from that of nymphs and adults.
Comparison of the diet among age sub-samples (Young instars, Nymphs and Adults) of Troglophilus cavicola and Troglophilus andreinii. Grey: green vegetables; light grey: fibres; black: arthropod remains.
Overlap analysis of food resource exploitation conducted in individuals of different age (young instars, nymphs and adults). The dendrograms were performed using euclidean distances based on the Morisita-Horn index matrices. (a: Troglophilus cavicola, b: Troglophilus andreinii).
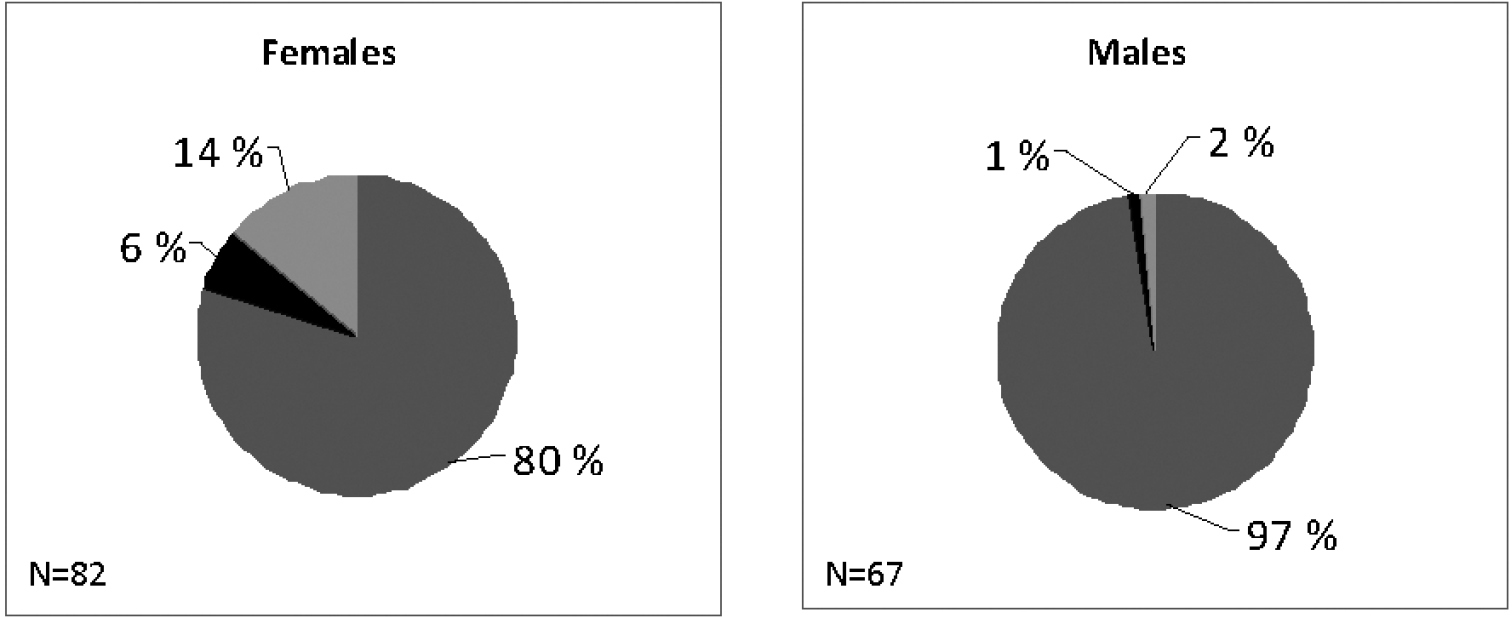
Differences in diet between males and females have been observed only in the Tranese population and only in a periodical sample. In this case, female niche breadth in autumn has a value of the Gini-Simpson index higher in comparison with that of males (i.e. 0.336 vs 0.04). This result seems related to the exploitation in this period of a consistent percentage of arthropod remains by females (Fig. 5).
Comparison of the autumnal diet between female and male sub-samples of Troglophilus andreinii. Grey: green vegetables; light grey: fibres; black: arthropod remains.
Remarkable differences in exploitation of these resources can be observed comparing the diet of the two populations herein studied. In particular, in the Troglophilus cavicola population we observed a diet mainly based on arthropod remains (69%). This finding is chiefly due to the exploitation inside the cave of this type of resource in winter during the strictly hypogean ecophase. In this case, individuals at Covoli di Velo were forced into the cave by the hard climate typical of pre-alpine winter. The large amount of arthropods in this unfavourable winter period can be related to the high availability of this resource inside the Covoli di Velo, where a complex cave biocenosis is present (
The analysis of niche breadth seems to confirm this result: lower values of Gini-Simpson index in Troglophilus cavicola indicating a dominance of a single type of resource (arthropods), are observed only in winter; in the same period, the Tranese population shows an higher value of this index, suggesting a diet more balanced among the available resources outside and inside the cave.
On the whole, as described for other Rhaphidophoridae species (
The study of overlap resource exploitation seems to indicate a separation of diet among young instars and the other age classes of the populations (nymphs and adults). This finding is confirmed by the Morisita–Horn index and is more evident in the Troglophilus cavicola populations. As found in the Italian Dolichopoda populations (
We thanks all of the individuals that collaborated with the researchers, and we are especially indebted to Federica De Bellis, Enrico Mezzanotte and Marco Sommaro for the help in the collection of the cricket samples and processing of our data and Roberta Salmaso for the assemblage of the pictures.
We are also grateful to James Tyler for the language revision.