Citation: Dörge DD, Zaenker S, Klussmann-Kolb A, Weigand AM (2014) Traversing worlds - Dispersal potential and ecological classification of Speolepta leptogaster (Winnertz, 1863) (Diptera, Mycetophilidae). Subterranean Biology 13: 1–16. doi: 10.3897/subtbiol.13.6460
Speolepta leptogaster (Winnertz, 1863) is frequently occurring in European subterranean environments. As for most cave animals, studies addressing non-anatomical aspects are sparse. Here we present the first molecular study on S. leptogaster. We investigated the demographic structure (i.e. COI locus) of 69 specimens from 36 underground populations in Hesse (Central German Uplands) to get first insights into the species’ dispersal ability.
In total, 14 haplotypes were revealed. Haplotype diversity was relatively high, whereas nucleotide diversity was low. Furthermore, a significant but low pattern of isolation-by-distance and (a) past population expansion event(s) were detected.
Our genetic results suggest a (good) active dispersal ability for Speolepta leptogaster. The occurrence of several surface records of adult specimens corroborates this hypothesis. We discuss the developmental stages of S. leptogaster in the context of the ecological classification system and regard the species as a eutroglophile. Evidence has been found to distinguish two larval types. A reconstructed life-cycle of the species is provided.
Cave animal, ecotone, phylogeography, mobility, ecological versatility
Century and a half have passed since the mycetophilid Speolepta leptogaster (Winnertz, 1863) (Diptera, Mycetophilidae) has been described. The species is widely distributed throughout Europe and can complete its entire life-cycle in subterranean environments such as caves, mines or related underground habitats. Originally placed into the genus Polylepta,
Here, we investigate the dispersal ability of Speolepta leptogaster by integrating population genetic data of specimens collected from underground sites in the Central German Uplands (Hesse). The study region was chosen since it was covered by permafrost during the Last Glacial Maximum (18, 000 – 24, 500 ybp) (
In total, 69 specimens of Speolepta leptogaster from 37 different caves, bunkers, wells, tunnels and cellars in Hesse (maximum of three per site) and one from a bunker in Poland were analyzed. The samples (Table 1) were collected and identified by members of the Hesse Federation for Cave and Karst Research (Germany) (
Dataset of analyzed specimens of Speolepta leptogaster and locality information. LS: life stage (L: larva, P: pupa, I: imago); H: haplotype. The geographic coordinates are in Degrees (°), Minutes (‘) and Decimal seconds (“). The locality numbers resemble the numbers given to each natural region by the German Bundesamt für Naturschutz.
| # | LS | Coordinates | Biotope | Locality numbers | H | NCBI |
|---|---|---|---|---|---|---|
| 1a | L | 50°10'13.80"N, 9°24'11.92"E | natural cave | 141 Sandsteinspessart | 9 | KF624625 |
| 1b | L | 50°10'13.80"N, 9°24'11.92"E | natural cave | 141 Sandsteinspessart | 1 | KF624626 |
| 1c | L | 50°10'13.80"N, 9°24'11.92"E | natural cave | 141 Sandsteinspessart | 4 | KF624627 |
| 2a | L | 50°15'49.43"N, 9°31'39.86"E | natural cave | 141 Sandsteinspessart | 1 | KF624628 |
| 2b | L | 50°15'49.43"N, 9°31'39.86"E | natural cave | 141 Sandsteinspessart | 1 | KF624629 |
| 3a | I | 50°4'55.99"N, 7°48'56.02"E | mine shaft | 304 Westlicher Hintertaunus | 1 | KF624630 |
| 3b | I | 50°4'55.99"N, 7°48'56.02"E | mine shaft | 304 Westlicher Hintertaunus | 1 | KF624631 |
| 4a | P | 50°5'34.37"N, 7°51'6.16"E | mine shaft | 304 Westlicher Hintertaunus | 8 | KF624632 |
| 4b | L | 50°5'34.37"N, 7°51'6.16"E | mine shaft | 304 Westlicher Hintertaunus | 8 | KF624633 |
| 5a | L | 50°6'36.32"N, 7°56'49.81"E | mine shaft | 304 Westlicher Hintertaunus | 2 | KF624634 |
| 5b | L | 50°6'36.32"N, 7°56'49.81"E | mine shaft | 304 Westlicher Hintertaunus | 2 | KF624635 |
| 5c | L | 50°6'36.32"N, 7°56'49.81"E | mine shaft | 304 Westlicher Hintertaunus | 2 | KF624636 |
| 6 | L | 50°9'19.87"N, 8°4'53.98"E | mine shaft | 304 Westlicher Hintertaunus | 1 | KF624637 |
| 7a | L | 50°52'40.73"N, 8°28'11.28"E | mine shaft | 320 Gladenbacher Bergland | 7 | KF624638 |
| 7b | L | 50°52'40.73"N, 8°28'11.28"E | mine shaft | 320 Gladenbacher Bergland | 7 | KF624639 |
| 8 | L | 50°53'21.05"N, 8°25'39.54"E | mine shaft | 320 Gladenbacher Bergland | 11 | KF624640 |
| 9 | L | 50°52'23.23"N, 8°28'37.34"E | rock cellar | 320 Gladenbacher Bergland | 3 | KF624641 |
| 10a | L | 51°0'4.61"N, 8°35'41.53"E | mine shaft | 332 Ostsauerländer Gebirgsrand | 2 | KF624642 |
| 10b | L | 51°0'4.61"N, 8°35'41.53"E | mine shaft | 332 Ostsauerländer Gebirgsrand | 2 | KF624643 |
| 10c | L | 51°0'4.61"N, 8°35'41.53"E | mine shaft | 332 Ostsauerländer Gebirgsrand | 2 | KF624644 |
| 11a | L | 51°23'34.73"N, 8°41'28.79"E | mine shaft | 332 Ostsauerländer Gebirgsrand | 1 | KF624645 |
| 11b | L | 51°23'34.73"N, 8°41'28.79"E | mine shaft | 332 Ostsauerländer Gebirgsrand | 14 | KF624646 |
| 11c | L | 51°23'34.73"N, 8°41'28.79"E | mine shaft | 332 Ostsauerländer Gebirgsrand | 1 | KF624647 |
| 12a | L | 51°13'56.89"N, 8°54'4.32"E | natural cave | 340 Waldecker Tafelland | 2 | KF624648 |
| 12b | L | 51°13'56.89"N, 8°54'4.32"E | natural cave | 340 Waldecker Tafelland | 2 | KF624649 |
| 13a | L | 51°14'25.08"N, 8°54'9.40"E | mine shaft | 340 Waldecker Tafelland | 1 | KF624650 |
| 13b | L | 51°14'25.08"N, 8°54'9.40"E | mine shaft | 340 Waldecker Tafelland | 1 | KF624651 |
| 14 | L | 51°18'55.58"N, 9°24'26.86"E | undercroft | 342 Habichtswälder Bergland | 1 | KF624652 |
| 15a | L | 51°7'41.70"N, 8°59'0.17"E | spring | 344 Kellerwald | 1 | KF624653 |
| 15b | L | 51°7'41.70"N, 8°59'0.17"E | spring | 344 Kellerwald | 1 | KF624654 |
| 16 | L | 51°7'32.66"N, 8°59'33.47"E | spring | 344 Kellerwald | 1 | KF624655 |
| 17 | L | 50°30'3.56"N, 9°7'25.43"E | rock cellar | 350 Unterer Vogelsberg | 1 | KF624656 |
| 18a | L | 50°31'0.59"N, 9°32'3.73"E | mine shaft | 350 Unterer Vogelsberg | 1 | KF624657 |
| 18b | L | 50°31'0.59"N, 9°32'3.73"E | mine shaft | 350 Unterer Vogelsberg | 1 | KF624658 |
| 19 | I | 50°27'0.29"N, 9°49'15.92"E | rock cellar | 353 Vorder- und Kuppenrhön | 2 | KF624659 |
| 20 | L | 50°34'21.18"N, 9°57'38.63"E | rock cellar | 353 Vorder- und Kuppenrhön | 12 | KF624660 |
| 21 | L | 50°35'23.96"N, 9°59'54.13"E | rock cellar | 353 Vorder- und Kuppenrhön | 1 | KF624661 |
| 22a | P | 50°35'57.73"N, 9°59'59.60"E | culvert | 353 Vorder- und Kuppenrhön | 1 | KF624662 |
| 22b | P | 50°35'57.73"N, 9°59'59.60"E | culvert | 353 Vorder- und Kuppenrhön | 1 | KF624663 |
| 23a | L | 50°30'30.64"N, 9°55'48.65"E | mine shaft | 354 Hohe Rhön | 1 | KF624664 |
| 23b | L | 50°30'30.64"N, 9°55'48.65"E | mine shaft | 354 Hohe Rhön | 6 | KF624665 |
| 23c | L | 50°30'30.64"N, 9°55'48.65"E | mine shaft | 354 Hohe Rhön | 6 | KF624666 |
| 24a | L | 50°28'7.25"N, 9°57'45.40"E | spring | 354 Hohe Rhön | 1 | KF624667 |
| 24b | L | 50°28'7.25"N, 9°57'45.40"E | spring | 354 Hohe Rhön | 1 | KF624668 |
| 25a | L | 50°52'35.18"N, 9°42'27.40"E | brick-built cellar | 355 Fulda-Haune-Tafelland | 1 | KF624669 |
| 25b | L | 50°52'35.18"N, 9°42'27.40"E | brick-built cellar | 355 Fulda-Haune-Tafelland | 1 | KF624670 |
| 26a | L | 50°53'4.56"N, 9°43'25.10"E | rock cellar | 355 Fulda-Haune-Tafelland | 1 | KF624671 |
| 26b | L | 50°53'4.56"N, 9°43'25.10"E | rock cellar | 355 Fulda-Haune-Tafelland | 1 | KF624672 |
| 27a | L | 50°51'35.21"N, 9°45'19.94"E | mine shaft | 355 Fulda-Haune-Tafelland | 1 | KF624673 |
| 27b | L | 50°51'35.21"N, 9°45'19.94"E | mine shaft | 355 Fulda-Haune-Tafelland | 13 | KF624674 |
| 27c | L | 50°51'35.21"N, 9°45'19.94"E | mine shaft | 355 Fulda-Haune-Tafelland | 1 | KF624675 |
| 28a | L | 51°7'34.36"N, 9°47'35.70"E | brick-built tunnel | 357 Fulda-Werra-Bergland | 1 | KF624676 |
| 28b | L | 51°7'34.36"N, 9°47'35.70"E | brick-built tunnel | 357 Fulda-Werra-Bergland | 10 | KF624677 |
| 29 | L | 51°12'26.24"N, 9°52'16.82"E | mine shaft | 357 Fulda-Werra-Bergland | 1 | KF624678 |
| 30 | L | 51°0'43.13"N, 9°55'45.73"E | mine shaft | 357 Fulda-Werra-Bergland | 1 | KF624679 |
| 31a | L | 51°0'24.88"N, 9°57'44.68"E | mine shaft | 357 Fulda-Werra-Bergland | 5 | KF624680 |
| 31b | L | 51°0'24.88"N, 9°57'44.68"E | mine shaft | 357 Fulda-Werra-Bergland | 5 | KF624681 |
| 31c | L | 51°0'24.88"N, 9°57'44.68"E | mine shaft | 357 Fulda-Werra-Bergland | 5 | KF624682 |
| 32a | L | 51°13'29.10"N, 9°57'8.68"E | mine shaft | 358 Unteres Werratal | 1 | KF624683 |
| 32b | L | 51°13'29.10"N, 9°57'8.68"E | mine shaft | 358 Unteres Werratal | 1 | KF624684 |
| 33a | L | 51°13'27.05"N, 9°57'11.74"E | touristic mine | 358 Unteres Werratal | 2 | KF624685 |
| 33b | L | 51°13'27.05"N, 9°57'11.74"E | touristic mine | 358 Unteres Werratal | 2 | KF624686 |
| 34a | L | 51°10'45.44"N, 10°4'15.28"E | mine shaft | 358 Unteres Werratal | 1 | KF624687 |
| 34b | L | 51°10'45.44"N, 10°4'15.28"E | mine shaft | 358 Unteres Werratal | 1 | KF624688 |
| 35 | L | 51°31'8.11"N, 9°22'39.43"E | bunker complex | 361 Oberwälder Land | 1 | KF624689 |
| 36a | L | 51°31'8.11"N, 9°22'39.43"E | bunker complex | 361 Oberwälder Land | 1 | KF624690 |
| 36b | L | 51°31'8.11"N, 9°22'39.43"E | bunker complex | 361 Oberwälder Land | 1 | KF624691 |
| 36c | L | 51°31'8.11"N, 9°22'39.43"E | bunker complex | 361 Oberwälder Land | 1 | KF624692 |
| 37 | I | 52°24'0"N, 15°31'59.99"E | bunker complex | Nietoperek (Poland) | 8 | KF624693 |
| Σ 69 |
A small piece of the posterior end of the body (approx. one sixth of the total animal) was used for larval samples. In the case of pupae and imagines, a larger portion of the abdomen (approx. ¼) was macerated. DNA isolation was performed according to the instructions of the DNEasy Blood & Tissue Kit (Qiagen Sample and Assay Technologies, Hilden, Deutschland) for the column purification of animal tissue.
A 662 bp fragment of the Cytochrome C Oxidase subunit 1 (COI) gene was amplified using the primers C1-J-2195 5’-TTGATTTTTTGGTCACCCTGAAGT-3’ and TL2-N-3014 5’-TCCAATGCACTAATCTGCCATATTA-3’ established by
The software DnaSP v5 (
A Mantel-test (
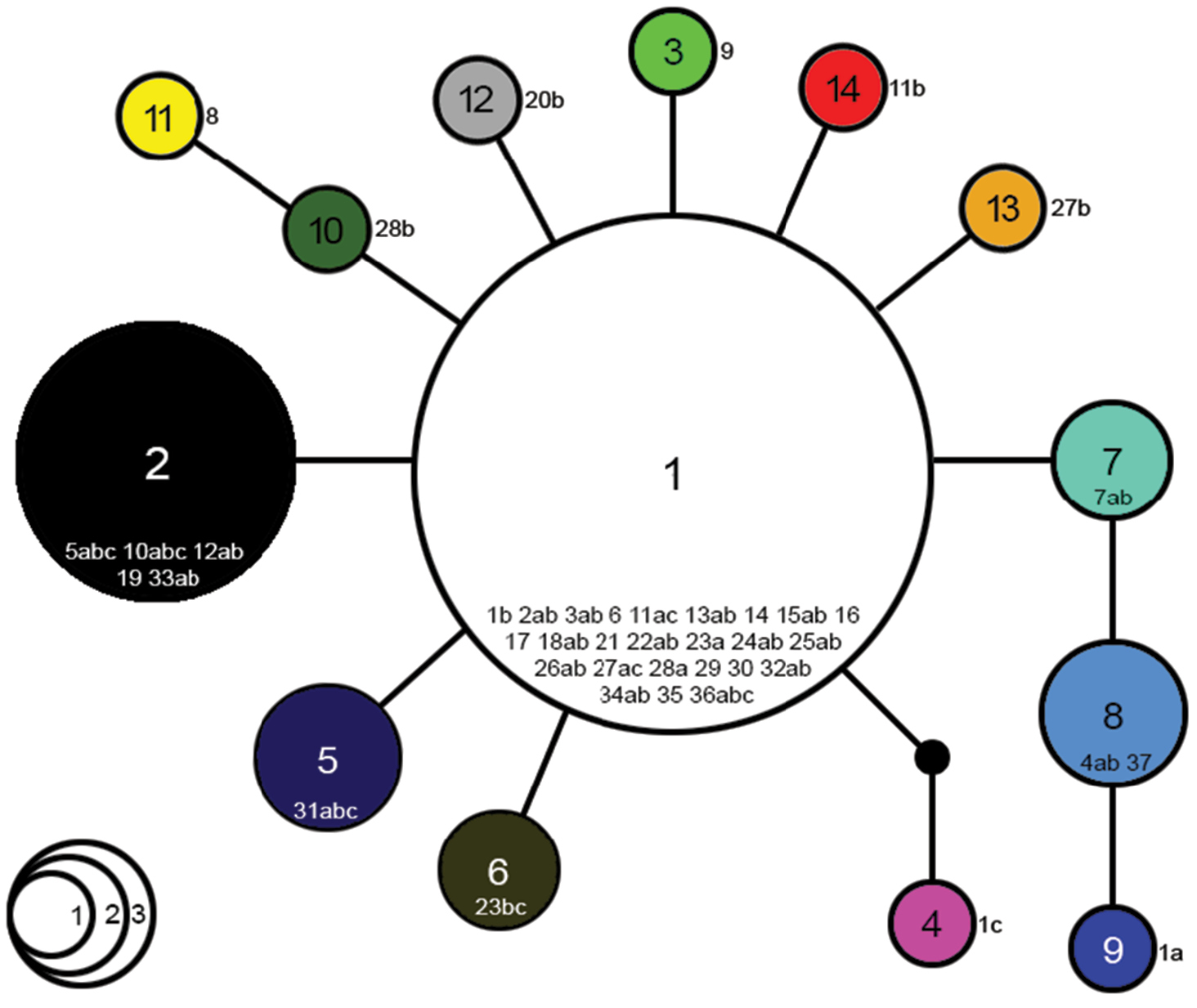
No gaps were present in the COI-alignment. In total, 14 haplotypes (H1-H14) were revealed. Haplotype diversity was high with a value of Hd = 0.7017, whereas nucleotide diversity was low with π = 0.00165 (Table 2). The haplotype network (Figure 1) indicated a highly frequent haplotype, H1, which consisted of 39 samples (57%) and a second relatively frequent haplotype, H2, which comprised 11 samples (16%). Eight haplotypes were singletons (H3, H4, H9-H14), two consisted of two (H6 and H7) and two of three samples (H5 and H8). There were only four haplotypes (H4, H8, H9 and H11) which were not directly connected to the most frequent haplotype H1.
CO1 haplotype network for Speolepta leptogaster. Haplotypes are numbered in sequence with their volume proportional to their frequency in the total dataset. Lines interconnecting the haplotypes illustrate the mutational course and the number of mutational steps between them. Numbers with letters within or alongside circles refer to Table 1.
Overview of statistical values. Estimates are given for neutrality tests (Fu’s Fs, Tajima’s D), haplotype (Hd), nucletide diversity (π) and Pearson’s r in a Mantel-test (geographic distance vs. genetic distance).
| estimates | value | p-value |
|---|---|---|
| Fu’s Fs | -10.28 | p < 0.001 |
| Tajima’s D | -1.89 | p = 0.004 |
| Hd | 0.7017 | |
| π | 0.00165 | |
| r | 0.17 | p < 0.0001 |
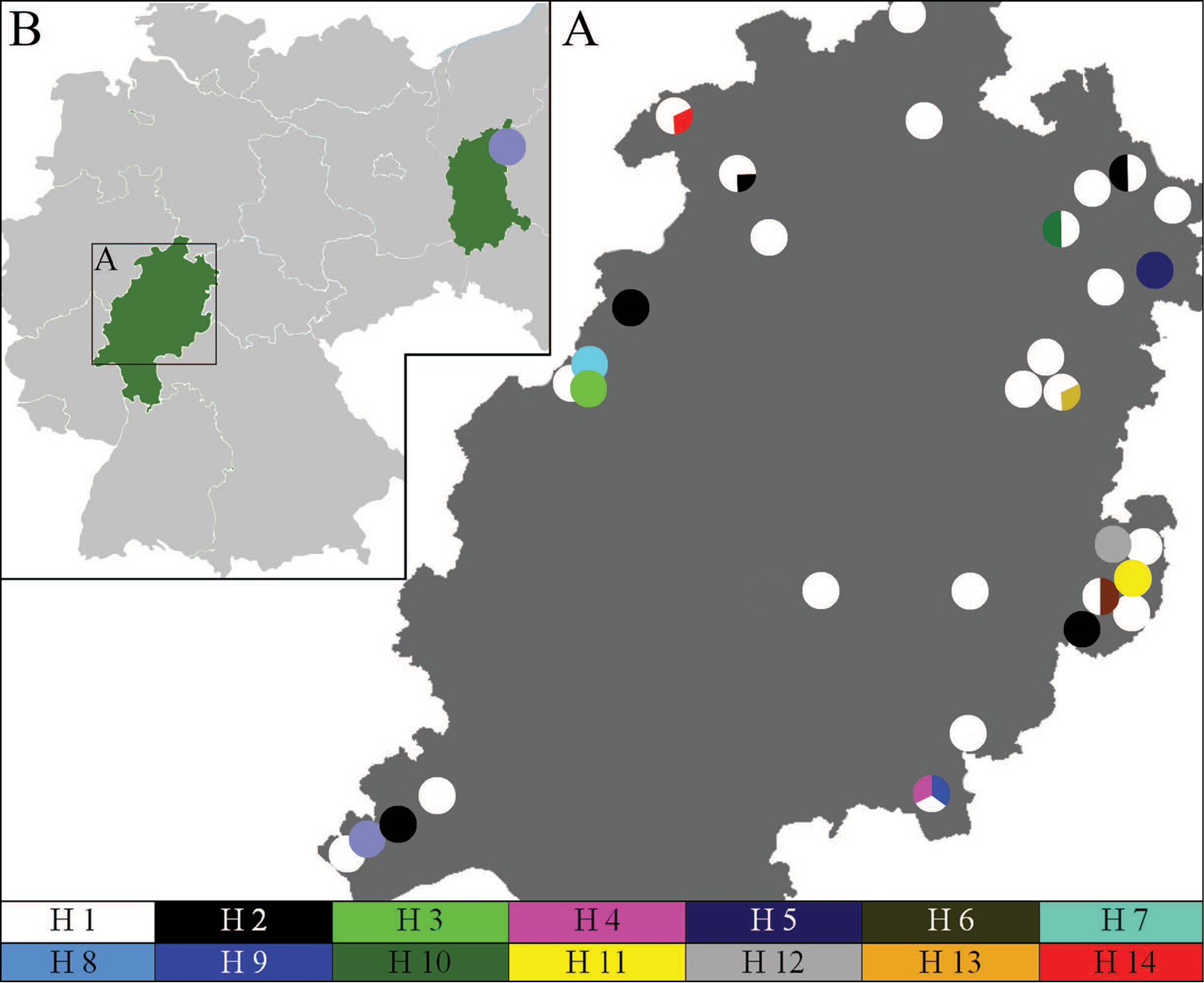
When the different haplotypes are placed in a geographical context (Figure 2), H1 can be revealed throughout the study region of Hesse. Haplotype 2 demonstrates a similar distribution except in the central underground sites and the most northern parts of Hesse. Haplotype 8 occurs once in southern Hesse and in the sampling site in Poland. All other haplotypes have been found within only a single locality. At a few localities several haplotypes co-occurred: In north-western Hesse H14+H1 and H2+H1; in north-eastern Hesse H9+H1, H2+H1 and H13+H1 and in south-eastern Hesse, H10+H6+H1.
Spatial pattern of haplotypes of Speolepta leptogaster in Hesse. The haplotype (H) distribution of Speolepta leptogaster within Hesse (A) with a comparison group in Poland (B) is depicted as a circle for every underground locality with colored sections for the different haplotypes. To be depicted in a reasonable manner, multiple localities were reduced to one circle if they were situated nearby (up to 4 km) and had the same color.
The results of both neutrality tests were significantly negative (p < 0.01) and point to (a) past population expansion event(s) (Table 2). In our dataset, 28.5% of all mutations (4/14) were non-synonymous leading to a change of the respective amino acid.
The Mantel-test (r = 0.17, p < 0.0001) reveals a significant positive low correlation between genetic distance and geographic distance (Table 2).
The region of the Central German Uplands (including our target area of Hesse) was covered with permafrost during the Last Glacial Maximum (18, 000 – 24, 500 years ago) (
Original description of Speolepta leptogaster (Winnertz, 1863). Translated from German. Originally this species has been described under the name of Polylepta leptogaster.
| The habitus is very similar to the Bolitophila. Body color brown. Mouth-rim slightly pulled forward and garlanded with hairs. The filamentous antennae are about 1.33 times as long as head and thorax together. The flagellum links are 3 to 4 times as long as broad. The haltere is whitish with a black-brown tip. The abdomen is very slim and cylinder-shaped, about 5 to 6 times as long as the very short thorax and constricted at the base. The coxa and femur yellow, tibia more brownish, tarsus light brown. The feet of the front legs are 2.33 times as long as the tibia, the tibia slightly shorter than the metatarsus (9 : 9 ¾) with lanceolate basis. Wings slightly greyish nearly colorless; the subcosta proceeding over the cubitus up to the tip of the wing, the supporting vein broken off in front of the lateral vein, the marginal lateral vein pulled far back, the discal cell trapezoid-shaped, 1.5–2 times as long as broad, the style of the upper fork about half the length of the upper prong, the basis of the rear cell under the middle of the wing on the far side of the discal cell, the axilla vein not sturdy, broken off on the opposite side of the rear cell. I only captured a female of this very rare species once in august in a swampy, forested area. A second female is located at the Royal Museum in Leyden, which differs from mine in the way that the discal cell, which is 1.5 times as long as broad in my specimen, is 2 times as long as broad. Apart from that, they completely matched. |
In general, our results imply good dispersal ability for Speolepta leptogaster. This is further supported by surface records of adult specimens compiled from the literature (Table 3). Although all developmental stages can be found in caves throughout the year, there are more findings of surface imagines in summer than in winter. Whether this is due to the biology of Speolepta leptogaster or to the smaller number of traps being laid out in winter cannot be determined.
Surface records of Speolepta leptogaster. N: number of specimens found, f: female, m: male.
| Region | Date of collection | Habitat/collection | N | Reference |
|---|---|---|---|---|
| swampy forest area | 1 f | |||
| Germany, Birgsau, südlich von Oberstdorf, im Stillachtal | 18–27 Sept. 1975 | light trap | 1 m | Plassmann (1977), first description Speolepta dissona |
| Germany, Saxon Switzerland 1.5 km SE of Obervogelgesang | 10–21 May 1997 | deciduous forest, Malaise trap | 1 f, 1 m | U. Kallweit (unpublished) |
| S-Germany, Grenzach | 11 May 2008 | 1 f | B. Rulik (unpublished) | |
| Germany, Harz mountains | 26 Oct. 2004 | natural mature spruce forest, yellow pan trap | 2 m | U. Kallweit (unpublished) |
| Germany, Harz mountains | 25 June 2004 | natural mature spruce forest, Malaise trap | 1 m | U. Kallweit (unpublished) |
| Germany, Hesse, Fulda, Mittelbergquelle | 10 Oct. 2004 | hand collection | 1 f | |
| Germany, Hesse, Auersbergquelle 21 | 18 Sept. 2009 | sweep net | 1 f | Zaenker unpublished |
| Germany, Hesse, Lützeler Sang-Quelle 2 | 25 Oct. 2007 | sweep net | 1 m | |
| Norway, Kvinnherad, Rosendal, riverside at Avlsgården, Baroniet | 11–15 May 1990 | Malaise trap | 1 f, 2 m | |
| Norway, Bergen, | 9 May–28 June 1991 | Malaise trap | 3 m | |
| Haukeland | ||||
| Norway, Bømlo, Vorland, Langevåg | 11 Feb 2002–12 Feb 2003 | Malaise trap | 7 f, 5 m | |
| Norway, Etne, Skånevik skyttarbane | 3 Sept. 2009 | sweep net | 1 f, 1 m | |
| Norway, Fjell, Vindenes | 5 Sept. 1978 | light trap | 1 f | |
| Norway, Os, Raudli | 23–30 May 1991 | 1 f, 1 m | ||
| Norway | June–Sept. 1991 | Malaise trap | 17 f, 11 m | |
| Norway, Os, Sæleli | 20–27 June 1991 | Malaise trap | 1 m | |
| Norway, Øygarden, Dalsvann, Alvøy | 5 June 1987 | light trap | 1 f | |
| Norway, Sveio, Førde, Solheimshaugen | 3–10 June 1991 | Malaise trap | 2 f | |
| Norway, Sveio, Førde, Solheimshaugen | 15 June 1991 | sweep net | 1 f, 1 m | |
| Norway, Sunndal, | 31 Mai–13 Jul. & 26 Aug.–6 Sept. 2004 | Malaise trap | 9 f, 14 m | |
| Jordalsgrenda, Jordalsøra, Hamrene | ||||
| Norway, Sunndal, | 14 June–3 Jul. & 12–25 Aug. 2005 | window trap | 1 f, 1 m | |
| Jordalsgrenda, Jordalsøra, Hamrene | ||||
| Norway, Aurland, Vassbygdvatnet | 4 Aug. 1969 | sweep net | 1 f | |
| (lower end of lake) | ||||
| Norway, Porsgrunn, Hitterødbekken | 13 June–11 Jul. 1988 | Malaise trap | 3 f | |
| Sweden, Jokkmokk, | 19–30 June 1968 | air suction trap | 1 f, 3 m | |
| Kaltisbäcken 1 km NNE Messaure | ||||
| Sweden, Lund, Høje Å (stream) at Värpinge | 23–28 May 2004 | yellow pan trap | 1 m | |
| Sweden, Klippan, | 26 Sept. 1983 | sweep net | 1 m | |
| Skäralid NR (ravine with stream) | ||||
| Sweden, Högsby kommun, | 29 Jul–31 Aug. 2004 | Malaise trap | 1 f | |
| Getebro | ||||
| Faroe Islands, Streymoy, | 13–17 Jul. 1990 | Malaise trap | 2 m | |
| Kvivik | ||||
| Czech Republic, Bohemia, Jizerské Hory Mts, Jedlový důl | 6–28 Jul. & 1–22 Sept. 2005 | Malaise trap | 3 m | |
| Czech Republic, Bohemia, Mt. Poledník | 29 Aug. - 5 Okt. 2004 | Malaise trap | 1 m | |
| Czech Republic, Bohemia, Krkonoše Mts., Bílé Labe | 16–30 Aug. 2007 | Malaise trap | 1 m | |
| Czech Republic, Bohemia, Labský důl | 24–27 Jul. 2006 | Malaise trap | 1 m | |
| Czech Republic, Moravia & Silesia, Hrubý Jeseník Mts, Velká kotlina | 9–26 June 2006 | Malaise trap | 1 m | |
| Czech Republic, Moravia & Silesia, Rejvíz | 20 May–1 Jul. 2005 | peat-bog, Malaise trap | 1 f | |
| Slovakia, Pol’ana Mts., Hrončecký grún | 7 May–4 Jul. 2006 | yellow pan trap | 1 m | |
| Slovakia, Kyslinky–Pod Dudášom | 15 June 2009 | 1 m | ||
| Slovakia, Predná Pol’ana Mt.–Bystré waterfall | 17 June 2009 | 1 m | ||
| Slovakia, Spády waterfall | 18 June & 4 Jul. 2009 | 2 m | ||
| [52 f, 64 m] |
Animals associated with underground habitats can be classified into different categories. Those classifications (i.e. ecological, behavioral, morphological) have been under constant change and revision. Multiple authors created new categories as well as split up old ones (Shiner 1854,
Since the biological diversity of subterranean species and ecology of subterranean habitats is high (
The larvae hatch and live solely within subterranean habitats reaching from microcavities to macrocaverns (or caves). They are incapable of surviving on the surface, which is related to their preference for a highly water-saturated atmosphere and adaptation to oxygen respiration. They lack a trachea system but are able to respire oxygen through a very thin cuticula spanning the entire body surface (
Conclusively, not all developmental stages are capable of surviving outside the subterranean zone. Thus, the completion of a life-cycle aboveground will be incomplete – eliminating the eutrogloxenes as a potential ecological category for Speolepta leptogaster. Due to the short life-span of adults rarely leaving the subterranean zone, the complete life-cycle (or a very large proportion) is within the subterranean environment – leaving the eutroglophiles and eutroglobionts. From an evolutionary point of view and for long-term survival, Speolepta leptogaster should be classified as a eutroglophile, i.e. being able to complete several underground generations but having the ability of surface dispersal. But pinpointing Speolepta leptogaster to a single ecological category clearly underestimates the ecological versatility of the species.
Even after 150 years, some facts about the biology of Speolepta leptogaster are still unknown. Specifically, these include the number of larval stages and the durations of the different life stages. Although
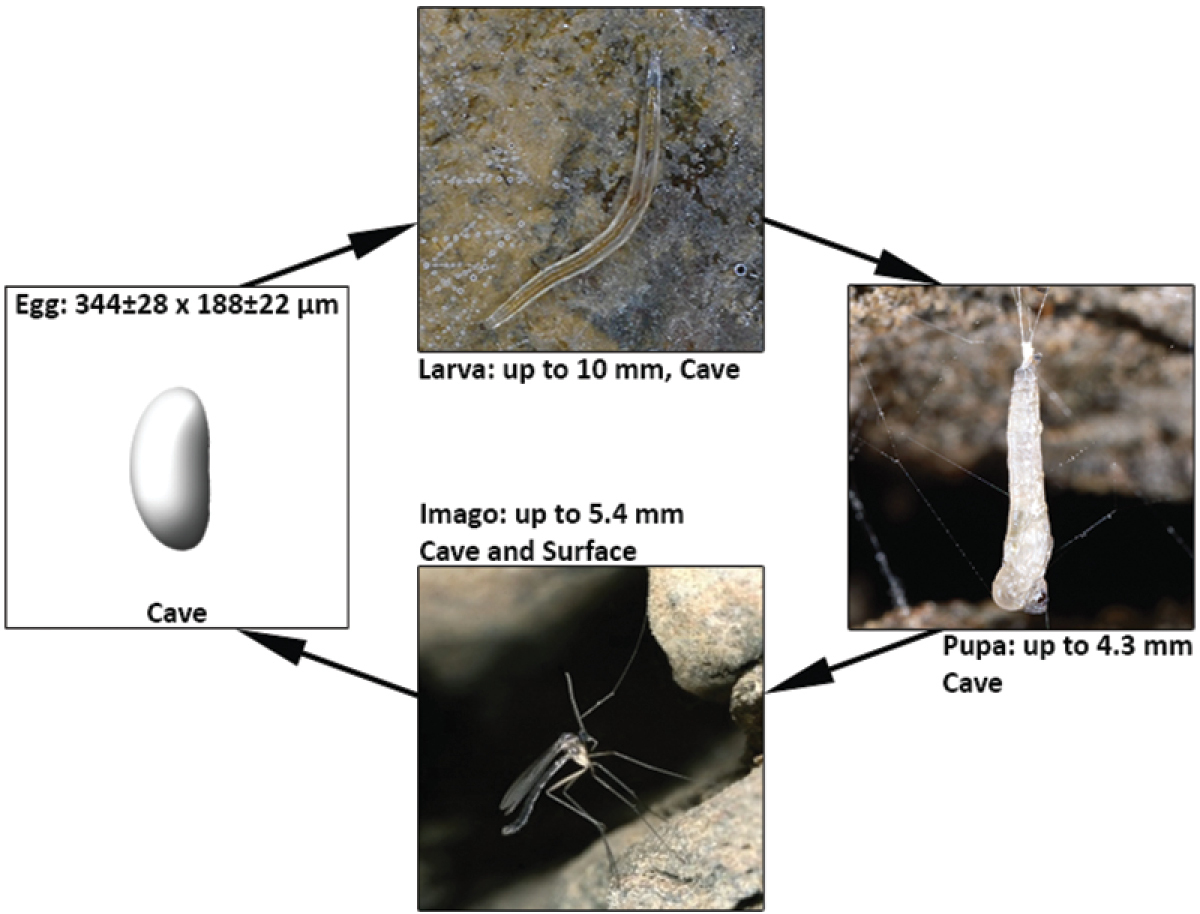
Potential life-cycle of Speolepta leptogaster; egg-drawing modified after the descriptions of
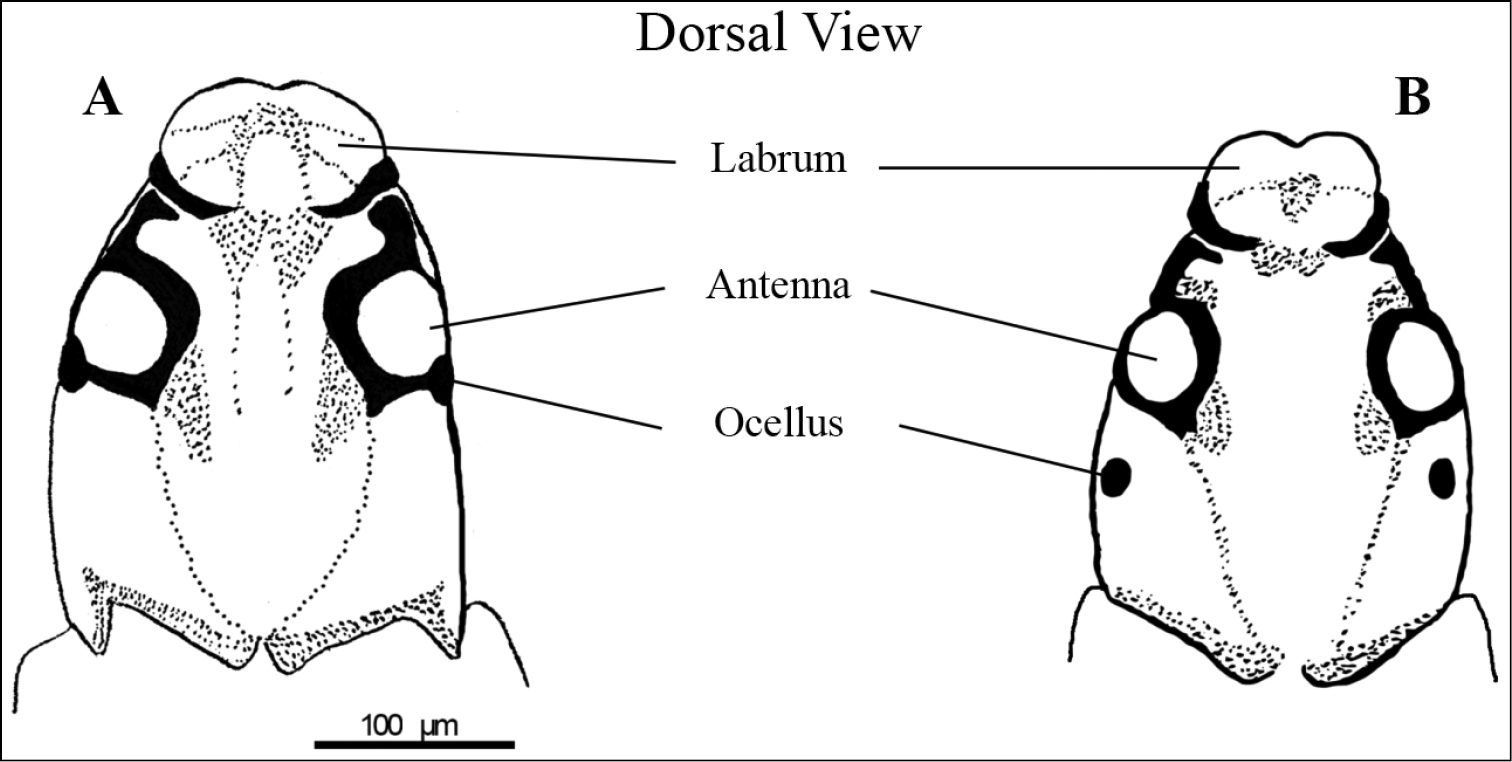
Two types of larvae were present in our material. They can be distinguished by a small deviance of the ocelli position and by body measurements (Figure 4). In type A, the ocellus is situated adjacent to the antenna whereas in type B, the ocellus is approximately 15 to 20 µm behind the antenna. The head capsule of larvae type B was thinner (A: about 210 µm, B: about 190 µm), slightly shorter (A: about 300 µm, B: about 280 µm) and generally more pointed than in type A. The labrum of larvae type B displays a deeper indentation in the middle of the frontal end. At the proximal end of the head area where the vermiform body begins, the larvae type A displays an edged transition, whereas with larvae type B, it is more semicircular. In the sample set, three larvae were found that appeared as depicted in type B. Two of which had only half the total length of the average length of a normal larva (4.7 mm and 5.0 mm compared to 10 mm). A third specimen was of normal size. Since different larval stages between hatching and pupating are reported in other Mycetophilidae (
Comparison between two larval stages of Speolepta leptogaster. A depicts the larger and probably older larva type A which on average is 10 mm long B depicts the smaller larva B type which is 5–10 mm long. Dotted regions depict areas of increased pigmentation, black regions illustrate maximum pigmentation.
Since this work presents the first step towards understanding the dispersal potential of the ecologically versatile species Speolepta leptogaster within a small area, subsequent studies should incorporate a larger, pan-European sampling to address diversification patterns for this abundant cave species. Its ecological diversity in congruence with frequent subterranean and sporadic surface animals further challenges the eco-categorical classification system applied for subterranean species.
Images of pupa and the male imago were taken by Klaus Bogon. We thank all members of the Hesse Federation for Cave and Karst Research for sample collection, Adrienne Jochum for proofreading the manuscript and two anonymous reviewers for their constructive comments.