Research Article |
|
Corresponding author: Leopoldo Ferreira de Oliveira Bernardi ( leopoldobernardi@gmail.com ) Academic editor: Stefano Mammola
© 2020 Leopoldo Ferreira de Oliveira Bernardi, Robson de Almeida Zampaulo, Marcus Paulo Alves de Oliveira.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Bernardi LFO, Zampaulo RA, Oliveira MPA (2020) A new species of Neocarus (Opilioacaridae) from a Brazilian ferruginous geosystem and notes on natural history. Subterranean Biology 36: 11-33. https://doi.org/10.3897/subtbiol.36.54034
|
Abstract
A new species of Neocarus is described from adult female and male specimens collected from an epigean and hypogean ferruginous geosystem located in southeast Brazil. The new species, Neocarus simmonsi sp. nov., possesses 15–17 ch-type palp setae, nude female pregenital Sternitogenital region, cylindrical ovipositor without setae, and a peculiar variation of setae in the genital and pregenital Sternitogenital region of the male, with smooth and tapering setae and/or barbed and tapering setae and/or stout and ribbed setae. Female genital setae are barbed, tapering and with a thin tip. Aspects of the ecology and life history of these mites are also presented.
Keywords
Acari, Biodiversity, Cave, Opilioacarida, Parasitiformes, South America
Introduction
The order Opilioacarida constitutes a cosmopolitan group whose distribution encompasses 26 countries (United States, Mexico, Belize, Cuba, Puerto Rico, Nicaragua, Costa Rica, Panama, Venezuela, Brazil, Argentina, Uruguay, Italy, Greece, Algeria, Angola, Gabon, Madagascar, Ivory Coast, Tanzania, South Africa, Yemen, Kazakhstan, India, Thailand and Australia) and all continents except Antarctica (e.g. Coineau and van der Hammen 1974;
Opilioacarida are edaphic mites found in a wide breadth of habitats, such as soils of forests or dry areas, in the midst of litter, under rocks or tree trunks, and in caves. Several of the species in Brazil have been collected in caves, in spite of not presenting morphological specializations for subterranean environments. However, it is still unclear whether these species have a preference for this type of environment or whether these records are due to the increased sampling effort directed to cave fauna in recent years (
Material and methods
Morphological study and gut content analysis
Specimens were collected during inventories of cave fauna, which involved thoroughly investigating caves for invertebrates under blocks, in accumulations of organic matter, and in fissures in the soil or cave walls (
Most of the material was studied as slide-mounted specimens. For this purpose, specimens were dissected, cleared in lactic acid and mounted on slides using Hoyer’s medium (
For the terminology for the palp tarsal sensilla we followed
Drawings were prepared using a Leica MDLS phase contrast microscope (Leica Microsystens, Wetzlar, Germany), connected to a drawing tube. Measurements were taken from adults using an ocular micrometer and are presented in micrometers (μm), average length is presented first, followed by length range in parentheses. Photos were taken with a 3.2 mega-pixel digital camera attached directly to a microscope.
Collection sites of the specimens examined were georeferenced using coordinates in degrees, minutes and seconds with the World Geodesic System (Datum WGS84).
All specimens are deposited in the following collections:
Abbreviations: F = female, M = male.
Biological remarks
Observations on seasonality were obtained from data produced by inventories of cave fauna in the municipalities of São Gonçalo do Rio Abaixo and Barão de Cocais, state of Minas Gerais, which used the method of active search as described above. The study was carried out in 109 cavities, with rainy season collections being made in January 2015 and dry season collections in October 2016.
Generalized linear models (GLMs), with contrast analysis were used to determine if there was a significant difference in the size of the Neocarus populations in the two seasonal campaigns. The models were built using abundance in each of the caves where Neocarus was observed as the response variable and each campaign as the explanatory variable. A negative binomial distribution with a log link functions was used since data presented significant overdispersion for Poisson error distribution (3.860). The GLM regression analysis was performed with R software (R Development Core Team 2019).
Taxonomic section
All specimens examined in this study are assigned to the genus Neocarus Chamberlin and Mulaik. Generic assignment is based on the following characteristics:
- adults with 3 ribbed and stout setae on the penultimate body segment;
- 4–6 foliate setae on the palp tarsus with no more than 5 lobes each;
- pectinate (d2) setae on palp absent;
- eupathidium (ζ1) in the main sensillar group of tarsus I and not crown like;
- shiny fleshy setae with a whip-like tip absent from the palps;
- dorsal segments VII to XVI and ventral segments X to XVI of the body without setae (according to
Klompen et al. 2015 ).
Arachnida Lamarck, 1802
Parasitiformes Reuter, 1909
Opilioacaridae With, 1902
Neocarus Chamberlin & Mulaik, 1942
Neocarus simmonsi sp. nov.
Diagnosis
Palp genu without p-type setae, tarsus with 15–17 ch-type sensilla and typical 6 pairs of foliate setae each with 1 small and thin lobe, plus 3 larger lobes with rounded, not filiform, tips. Sexual dimorphism in setation of prodorsal shield absent. Sternal setae St2 and St3 with attenuate tips. Pregenital area in female nude, and genital area with 6–12 barbed, tapering setae with thin tip. Pregenital areas in male with 1–6 stout, ribbed, and relatively blunt-tipped setae and 3–7 smooth/light barbed and tapering setae; genital area with 0–6 stout, ribbed, relatively blunt-tipped setae and 4–7 smooth/light barbed and tapering setae. Ovipositor nude, simple tube-like structure, without terminal setiform sensilla or lobes.
Description
Based on 13 females and 6 males.
Gnathosoma. Chelicera (Fig.
Subcapitulum (Figs
Palp (Figs
Idiosoma. Color: Violet-blue with the usual banding pattern. Color observed for live and alcohol preserved specimens (Fig.
Dorsum. Prodorsal shield with two pairs of lateral eyes. One pair of prodorsal lyrifissures present. Chaetotaxy in females and males consisting of, respectively, 186–204 and 218–242 setae. Sexual dimorphism in anterior portion of prodorsal shield absent. Dorsal idiosoma between the prodorsal shield and the preanal segment without setae, but with numerous lyrifissures arranged in transverse rows. Setation preanal segment limited to 1 dorsal, and 2 ventro-lateral setae. Anal valves with 7–12 stout, ribbed setae (9–12 in females; 7–9 in males).
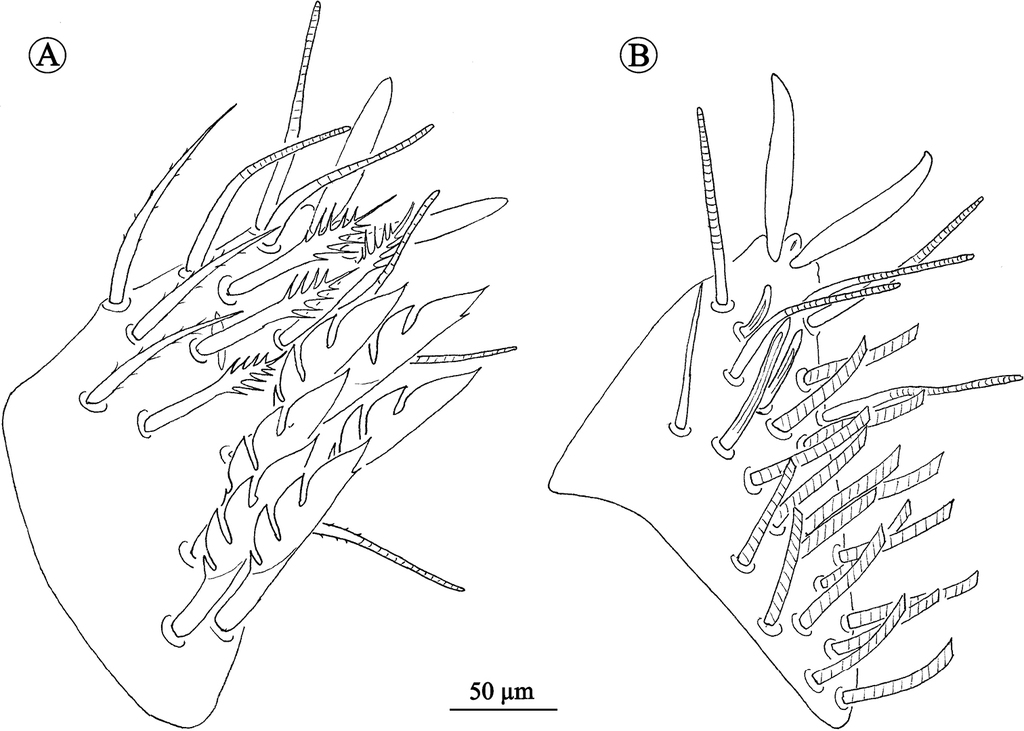
Sternitogenital region (Figs
Legs. Length of legs I–IV in females and males very similar, combined as “adults”, 3884 (3619–3941), 1973 (1812–2411), 1906 (1652–2047) and 3065 (2741–3520). Eupathidium z1 on tarsus I inserted in dorsal sensory field; simple, without enlarged tip. Solenidion wa on legs II positioned on acrotarsus; wa on tarsi III–IV absent. Solenidion wd on basitarsi II–IV inserted apically, not sunk into the segment. Ambulacra II–IV in adults with 2 smooth and attenuate setae (d and l). Ventral portion of acrotarsi II–IV with 3 pairs of setae; lateral portion with 2 pairs distinctly lateral, plus 1 pair of ventro-lateral and one pair of dorso-lateral setae. Setae lv of acrotarsi II–IV with one small barb. Papilliform setae on dorsal portion of the basitarsi II–III present. On leg I in both sex, thin and smooth setae restrict to telotarsus and distal portion of basitarsus. Coronidia present on basitarsi II–IV of all adults (basitarsus II 8–14; III 10–16, IV 13–19), absent on tibiae and genua II–IV.
Neocarus simmonsi sp. nov., view of sternitogenital Sternitogenital region; View of female sternitogenital Sternitogenital region (A) and view of variotion on genital Sternitogenital region (B); View of male sternitogenital Sternitogenital region (C) and view of variation on pregetial and genital Sternitogenital region (D).
Material examined
Type depository: Holotype female,
Paratypes
: 2 female and 1 male specimens deposited at
Etymology
The specific name is in honor of George C. Simmons due his contributions to studies on caves genesis and mineralogy. Simmons produced seminal papers in iron ore caves and karst in Brazil and one of his important research projects was conducted in cave MDIR_0020 (Simmons cave), near the type locality of the new Neocarus species.
Comparative notes
Neocarus simmonsi differs from N. potiguar Bernardi, Zacarias & Ferreira, 2012, N. proteus Bernardi, Klompen, Zacarias & Ferreira, 2013, and N. platensis (Silvestri, 1905) from Brazil by the absence of pregenital setae in the female. It differs from N. coronatus Araújo & Feres, 2018 by the presence of 6 (vs. 4) foliate setae on the palp tarsus; from N. potiguar, N. caipora Bernardi, Klompen & Ferreira, 2014, N. platensis (from Argentina and Uruguay) and N. misiones Vázquez, Bernardi & Klompen, 2020 by the absence (vs. presence) of p-type setae on the palp genu (N. proteus and N. spelaion Bernardi, 2018 are somewhat intermediate as they carry small numbers (1–7) of p-type setae on the palp genu), and from N. spelaion by the uniformity (vs. variability) in shape of the pregenital and genital setae in the male. N. simmonsi differs from N. entrerios Vázquez, Bernardi & Klompen, 2020 and N. coronatus by the absence (vs presence) of sexual differentiation in the setae on the prodorsal shield.
The ovipositor is a unique structure and its characteristic is useful to differentiate Opilioacarida species. Neocarus simmonsi presents an ovipositor cylindrical with a rounded tip similar only to N. potiguar, but differentiates from the species with terminal lobes, such as N. misiones (three small and very distinct roundish terminal lobe), N. entrerios (with a distinct pair of papillate hooks), N. spelaion (with a rounded and distinct lobes) and N. proteus (two rounded structures plus three membranes at tip. Neocarus coronatus and N. caipora differs from all South American species due the presence of setae on ovipositor. Neocarus platensis and N. ojastii have a poorly described ovipositor structure.
Life history remarks
Development and morphological abnormalities
The ability to regenerate appendages can be a great advantage for species of Opilioacarida since these organisms easily lose their appendages during different stages of development or even in adulthood (
It is not possible to say how many molts an adult can experience even after reaching sexual maturity, but the size of a male and female collected during field study suggests that growth may continue, and thus there can be more than one molt in adulthood. This is based on the observation that collected adult specimens exhibited the molting process, with two specimens (1 male and 1 female) having an old integument (exuvia), which was covering the new one. The new integument presented some characters already 9 to 18% larger than in any of the other adults collected. Based on the measurements of body setae,
Comparative setal patterns and shape for the pregenital and genital Sternitogenital region, ovipositor and palp of Neocarus adults.
| OCCURENCE | SPECIES/SUBSPECIES | FEMALE | MALE | Palp | |||
| Pregenital Sternitogenital region | Genital / Eugenital Sternitogenital region | Pregenital Sternitogenital region | Genital Sternitogenital region | ch-type | d-type | ||
| No. and type of setae | No. and type of setae | No. and type of setae | No. and type of setae | ||||
| North America | |||||||
| USA | Neocarus texanus | 2 st/r | nude | 4–6 st/r | 8–9 sh | 10–14(21*) | 5 |
| Mexico | Neocarus nohbecanus | nude | nude | 4–5 st/r | 5–7st/r | 17–19 | 4 |
| Mexico | Neocarus siankaanensis | nude | nude | 2 st/r | 4 st/r | 14–15 | 5 |
| Mexico | Neocarus bajacalifornicus bajacalifornicus | 2 st/r | nude | 5–8(13a) st/r | 7–8(11a) st/r | 14–18 (21a) | 5 |
| Mexico | Neocarus bajacalifornicus chamelaensis | 2–3 st/r | nude | 4–5 st/r | 4–6 st/r | 16 | 5 |
| Mexico | Neocarus calakmulensis | 2–3 st/r | nude | 2–6 st/r | 3–8 st/r | 17 | 5 |
| Mexico | Neocarus veracruzensis | 2 st/r | nude | 6–8 st/r, 0–1 s | 6–8 st/r | 13 | 5 |
| Mexico | Neocarus comalensis | 5–7 st/r | 3 st/r | 14–18 | 5 | ||
| Mexico | Neocarus chactemalensis | nude | nude | 4–6 st/r | 4–6 st/r | 11–13 | 4 |
| Central America | |||||||
| Nicaragua | Neocarus nicaraguensis | 2–5 st/r | nude | 2–7 st/r | 3–6 st/r | 18–22 | 5 or 6 |
| Cuba | Neocarus orghidani | nude | nude | 4–5 st/r | 5–7 st/r | 20–24 | 4 |
| Belize | Neocarus belizensis | nude | nude | 2–3 st/r | 4–5 st/r | 17–21 | 5 or 6 |
| South America | |||||||
| Venezuela | Neocarus ojastii** | nude | nude | 6–9? | 13 st/r | – | – |
| Brazil | Neocarus proteus | 2–5 st/r | 4–6 wb | 2–5 st/r | 3–5 sh | 12 or 13 | 5 or 6 |
| Brazil | Neocarus potiguar | 1 tp/r | 4–8 sh | 5 st/r | 7–10 st/r | 25–27 | 5 or 6 |
| Brazil | Neocarus coronatus | nude | 6 tp/b | 1–7 st/r | 5–15 tp/r | 18–25 | 4 |
| Brazil | Neocarus caipora | nude | 8–12 sh | 4–8 st/r | 5–8 tp/r | 15–16 | 6 |
| Brazil | Neocarus spelaion | nude | 10–12 sh | 9–12 sh or tp/r | 7–11 sh and/or tp/r | 14–18 | 5 or 6 |
| Brazil | Neocarus simmonsi | nude | 6–12 st/b or tp/b | 4–10 sh or tp/r or st/r | 4–10 sh or tp/r or st/r | 15–17 | 6 |
| Brazil/Argentina/Uruguay | Neocarus platensis | 0–2 st/r | 6–9 sh | 6–10 st/r | 5–10 sh | 14 | 5 or 6 |
| Argentina | Neocarus misiones | nude | 12–13 tp/b | 7–9 st/r and/or tp/b | 12–13 tp/b | 15 | 6 |
| Argentina | Neocarus entrerios | nude | 6–12 sh | 6–10 st/r | 8–10 tp/r | 20 | 6 |
Another interesting observation made on an adult male of N. simmonsi was the presence of morphological abnormalities of the palp, with the reduction and reorganization of setae as well as changes in the shape of the structure itself (Fig.
Gut contents
The gut contents of specimens of N. simmonsi includes unidentified plant fragments, arthropod remains, pollen, and fungal hyphae (Fig.
In addition to these materials, the stomach contents of three specimens evaluated under microscopy possessed remains of integuments (exuvia) of Opilioacarida (Fig.
During the observation of N. proteus kept laboratory condition in 2012, for about 4 months, also allowed documenting specimens feeding on their own exuvia after molting (2 observations) and even exuvia of other dead individuals (2 observations). Such laboratory data, associated with the observations of stomach contents of specimens of N. simmonsi in the present study and the absence of records of cannibalism among Opilioacarida bred and maintained in the laboratory (
Distribution, habitat, and seasonality
Neocarus simmonsi does not have morphological characteristics arising from isolation in subterranean environments and was found both in caves and the epigean environment. The only species whose morphology has been modified to the point of being considered troglobitic are Siamacarus dalgeri and S. withi, described by
The distribution of N. simmonsi extends across the entire Serra do Tamanduá and Dois Irmãos (municipalities of São Gonçalo do Rio Abaixo and Barão de Cocais) in a strip of at least 12 km. However, the effective distribution of the species is expected to be much greater, considering that it is a common species in the sampled area and that it is not restricted to subterranean environments (caves) (Figs
The study area is located northeast of the Quadrilátero Ferrífero (Iron Quadrangle), in an area of Atlantic Forest near the transition zone with the Cerrado biome (Brazilian savanna). The vegetation cover consists of forest formations and “campos rupestres” (rupestrian fields) at higher altitudes and shallow soils rich in metals (e.g. iron). Since it is a geomorphologically diversified area and located close to the transition between two biomes, there is a great diversity of flora and fauna. The Sternitogenital region, therefore, is considered a priority area for conservation in the state of Minas Gerais with “extreme biological importance” due to the high floristic and faunal richness and the presence of several endemic and endangered species (Drummond et al. 2005). There are a few hundred registered caves in the study area including the largest caves in the Quadrilátero Ferrífero.
The type locality, Simmons' cave, was mapped in 1960 and is one of the few examples of caves formed by dissolution in the Sternitogenital region. The cave is located at approximately 1140 meters above sea level and has only one entrance, aphotic zones, different compartments (halls), perennial lakes, and 146 meters of horizontal projection. With regard to organic resources, litter deposits were observed in the entrance area, along with guano of carnivorous and hematophagous bats. Lichens and fungi were observed on the walls and floor near the entrance to the cavity.
Neocarus simmonsi is an abundant species in caves in the Sternitogenital region, having been found in 73 of the 109 studied caves. The abundance of specimens was significantly higher in the wet season (estimated β ± S.E. = 1.29328 ± 0.22096; z = 5.853; p-value = 4.83e–9), ranging from few to dozens of individuals as the case of BRU_0012 cave (n = 48 during the wet season and n = 0 during the dry season), BRU_0023 cave (n = 21 during the wet season and n = 1 during the dry season), and BRU_0002 cave (n = 15 in the wet season and n = 1 during the dry season). This great oscillation may be explained by the fact that the number of individuals observed increases during the rainy season, or else it may be due to the migratory behavior of the species (Fig.
Average sampled individuals of Neocarus simmonsi sp. nov. per cave according to seasonality. The abundance values are significantly different between the two seasons according to the Generalized linear model analysis (z = 5.853; p-value = 4.83e–9) In the boxplots, the turquoise areas refer to the interquartile range around the observed median (central black line) and vertical bars represent the maximum-minimum range (excluding outliers).
In some caves individuals of N. simmonsi were found aggregated in groups of up to eight and ranging from protonymph stages to adult males and females. The species’ behavior is similar to that observed for N. caipora and Caribeacarus brasiliensis (
Final remarks
The number of described species of Opilioacarida from collections in karst areas and caves in Brazil is increasing (
Brazil stands out for its diversity of described species of Opiliacarida (11 spp.) and its great potential for new records in view of its territorial extension and the large concentration of caves in different lithologies and biomes (
Mites and other soil invertebrates generally exhibit seasonal fluctuations, with their richness and abundance being determined by environmental factors such as precipitation and temperature (
Opilioacarida is a group of species with interesting feeding habits. The material ingested by them is composed of large fragments of solid material of vegetable, animal and/or microbiological origin (
Finally, the order Opilioacarida always occupies a prominent position when referring to the study of development among mites. The development of these taxa comprises an embryonic phase (prelarva), subsequent larva, protonymphs, deutonymphs, tritonymphs and adults, but its growth can continue beyond the adult phase. The regeneration of appendages in adult individuals, however, is a peculiarity known only in this group among Parasitiformes and is rarely observed among a few Acariformes and arachnids in general (
Acknowledgements
We would like to thank Dr. Paulo Rebelles Reis for the incentive and for allowing the use of the equipment present in EPAMIG/CTSM–EcoCentro Lavras. Also, thanks to the speleologist group Spelayon Consultoria, BioEspeleo Consultoria Ambiental and Ativo Ambiental that provided all the specimens and cave information. This research received promotion and financial support from VALE S.A (Executive Board of Environmental Licensing and Speleology). LFOB thanks Coordination for the Improvement of Higher Education Personnel for providing a post-doctoral scholarship from the National Postdoctoral Programme (CAPES-PNPD/Brazil). Finally, we thank Rodrigo Lopes Ferreira, Marcel Santos de Araújo, and an anonymous referee for a detailed review of the manuscript.
References
- Araújo MS, Bichuette ME, Bauchan GR, Ochoa R, Feres RJF (2018a) A new species of cave dwelling Neocarus (Acari: Opilioacaridae) from Bahia state, Brazil, with remarks on taxonomic characters. Zootaxa 4402(2): 303–322. https://doi.org/10.11646/zootaxa.4402.2.4
- Araújo MS, Di Palma A, Feres RJF (2018b) A new species of Opilioacarus With, 1902 (Acari: Opilioacaridae) from Italy, and a new diagnosis of the genus. Zootaxa 4500(1): 135–145. https://doi.org/10.11646/zootaxa.4500.1.9
- Badejo MA (1990) Seasonal abundance of soil mites (Acarina) in two contrasting environments. Biotropica 22: 382–390. https://doi.org/10.2307/2388555
- Bento DM, Ferreira RL, Prous X, Souza-Silva M, Bellini BC, Vasconcellos A (2016) Seasonal variations in cave invertebrate communities in the semiarid Caatinga, Brazil. Journal of Cave and Karst Studies 78: 61–71. https://doi.org/10.4311/2015LSC0111
- Bernardi LFO, Borges-Filho EL (2018) Neocarus spelaion sp. n. (Parasitiformes, Opilioacaridae), a new species of cave dwelling Neocarus from Minas Gerais state, Brazil. Subterranean Biology 27: 1–16. https://doi.org/10.3897/subtbiol.27.25777
- Bernardi LFO, Zacarias MS, Ferreira RL (2012) A new species of Neocarus Chamberlin & Mulaik, 1942 (Acari: Opilioacarida) from Brazilian caves and karst areas. Zootaxa 68: 53–68. https://doi.org/10.11646/zootaxa.3416.1.5
- Bernardi LFO, Klompen H, Ferreira RL (2013a) Adult growth in Opilioacaridae With 1904 (Acari: Parasitiformes: Opilioacarida). Annals of the Entomological Society of America 1904: 788–790. https://doi.org/10.1603/AN13056
- Bernardi LFO, Klompen H, Zacarias MS, Ferreira RL (2013b) A new species of Neocarus Chamberlin & Mulaik, 1942 (Opilioacarida, Opilioacaridae) from Brazil, with remarks on its postlarval development. ZooKeys 358: 69–89. https://doi.org/10.3897/zookeys.358.6384
- Bernardi LFO, Silva FAB, Zacarias MS, Klompen H, Ferreira RL (2013c) Phylogenetic and biogeographic analysis of the genus Caribeacarus (Acari: Opilioacarida), with description of a new South American species. Invertebrate Systematics 27: 294–306. https://doi.org/10.1071/IS12041
- Bernardi LFO, Klompen H, Ferreira RL (2014) Neocarus caipora, a new species (Parasitiformes: Opilioacarida: Opilioacaridae) from brazilian Amazon caves. Acarologia 54: 47–56. https://doi.org/10.1051/acarologia/20142113
- CECAV (2019) CECAV/ICMBio: Centro Nacional de Pesquisas e Conservação em Cavernas/Instituto Chico Mendes de Conservação da Biodiversidade. https://www.icmbio.gov.br/cecav/publicacoes/96-anuario-estatistico-do-patrimonio-espeleologico-brasileiro-2018.html
- Coineau Y, Legendre R (1975) Sur un mode de regeneration appendiculaire inedit chez les Arthropodes: Ia regeneration des pattes marcheuses chez les Opilioacariens (Acari: Notostigmata). Comptes Rendus de l’Académie des Sciences 280: 41–43.
- Coineau Y, van der Hammen L (1979) The postembryonic development of Opilioacarida, with notes on new taxa and on a general model for the evolution. In: Piffl E (Ed.) Proceedings 4th International Congress of Acarology. Saalfelden, 437–441.
- Culver DC (1982) Cave Life. Harvard University Press, Massachusetts and London, 189 pp.
- Das NPI, Bastawade DB (2006) The first report of the Acarina suborder Opilioacarida from India, with description of new genus Indiacarus and a new species Indiacarus pratyusshi. Acarologia 47: 3–11.
- Dondale CD (1965) A spider’s first meal after molting. The Canadian Entomologist 97: 446–448. https://doi.org/10.4039/S0008347X00054821
- Drummond GM, Martins CS, Greco MB, Vieira F (2009) Biota Minas: Diagnóstico do Conhecimento sobre a Biodiversidade no Estado de Minas Gerais – Subsídio ao Programa Biota Minas. Fundação Biodiversitas, Belo Horizonte. Fundação Biodiversitas, 624 pp.
- Ferreira RL, Martins VM, Paixão EA, Silva MS (2015) Spatial and temporal fluctuations of the abundance of Neotropical cave-dwelling moth Hypena sp. (Noctuidae, Lepidoptera) influenced by temperature and humidity. Subterranean Biology 16: 47–60. https://doi.org/10.3897/subtbiol.16.5137
- Freitas CR, Littlejohn RN (1987) Cave climate: assessment of heat and moisture exchange. Journal of Climatology 7: 553–569. https://doi.org/10.1002/joc.3370070604
- Furumizo RT, George WW (1976) A case of postimaginal molt in the American house dust mite Dermatophagoides farinae Hughes 1961 (Acari: Pyroglyphidae). Acarologia 17: 730–733.
- Grandjean F (1936) Un acarien synthétique: Opilioacarus segmentatus With. Bulletin de la Société d’Histoire Naturelle de l’Afrique du Nord 27: 413–444.
- Imamura T (1952) Notes on the moulting of the adult of the water mite, Arrenurus uchidai n. sp. Annotationes Zoologicae Japonense 25: 447–451.
- Johnson NF (2010) Future taxonomy today: new tools applied to accelerate the taxonomic process. In: Polaszek A (Ed.) Systema Naturae 250: The Linnaean Ark. CRC Press Taylor and Francis Group, 137–147. https://doi.org/10.1201/EBK1420095012-c13
- Juvara-Bals I, Baltac M (1977) Deux nouvelles espèces d’Opilioacarus (Acarina: Opilioacarida) de Cuba. In: Orghidan T, Jiménez NA, Decou V, Negrea S, Bayés NV (Eds) Résultats des expéditions biospéleogiques Cubano-Roumaines á Cuba. Academiei Republicii Socialiste Romania, Bucuresti, 169–184.
- Klompen JSH (2000) Prelarva and larva of Opilioacarus (Neocarus) texanus (Chamberlin and Mulaik) (Acari: Opilioacarida) with notes on the patterns of setae and lyrissures. Journal of Natural History 34: 1977–1992. https://doi.org/10.1080/00222930050144819
- Klompen H, Vázquez MM, Bernardi LFO (2015) Post-embryonic development in the mite suborder Opilioacarida, with notes on segmental homology in Parasitiformes (Arachnida). Experimental and Applied Acarology 67: 183–207. https://doi.org/10.1007/s10493-015-9939-7
- Leclerc P (1989) Considérations paléobio-géographiques a propos la découverte en Thailande d’Opilioacariens nou-veaux (Acari – Notostigmata). Compte Rendu des Séances, Société de Biogéographie, Paris 65: 162–74.
- Lewinsohn TM, Prado PI (2005) How many species are there in Brazil? Conservation Biology 19: 619–624. https://doi.org/10.1111/j.1523-1739.2005.00680.x
- Mammola S, Isaia M (2018) Day-night and seasonal variations of a subterranean invertebrate community in the twilight zone. Subterranean Biology 27: 31–51. https://doi.org/10.3897/subtbiol.27.28909
- Michener CD (1946) The taxonomy and bionomics of some panamanian trombidiid mites (Acarina). Annals of the Entomological Society of America 39: 349–380. https://doi.org/10.1093/aesa/39.3.349
- MMA (2009) MMA – Ministério do Meio Ambiente/Instituto Chico Mendes de Conservação da Bodiversidade (2012) Instrução Normativa MMA N° – 2, de 20 de agosto de 2009, Brazil. http://www.icmbio.gov.br/cecav/images/download/IN%2002_MMA_Comentada.pdf
- MMA (2017) MMA – Ministério do Meio Ambiente/Instituto Chico Mendes de Conservação da Bodiversidade 169 Diário Oficial da União Instrução Normativa MMA N° – 2, de 30 de agosto de 2017, Brazil. http://www.in.gov.br/autenticidade.html
- Monte BGO, Bichuette ME (2020) Taxonomic distinctness of the subterranean fauna from Peruaçu Caves National Park, state of Minas Gerais, eastern Brazil. Biota Neotropica 20: 1–20. https://doi.org/10.1590/1676-0611-bn-2019-0810
- Oliveira U, Paglia AP, Brescovit AD, Carvalho CJB, Silva DP, Rezende DT, Leite FSF, Batista JAN, Barbosa JPPP, Stehmann JR, Ascher JS, Vasconcelos MF, Marco JR PM, Lowenberg-Neto P, Dias PG, Ferro VG, Santos AJ (2016) The strong influence of collection bias on biodiversity knowledge shortfalls of Brazilian terrestrial biodiversity. Diversity Distribution 22: 1232–1244. https://doi.org/10.1111/ddi.12489
- R Development Core Team (2016) A language and environment for statistical computing. R Foundation for Statistical Computing.
- Silva MS, Martins RP, Ferreira RL (2011) Trophic dynamics in a neotropical limestone cave. Subterranean Biology 9: 127–138. https://doi.org/10.3897/subtbiol.9.2515
- Simon KS, Pipan T, Culver DC (2007) A conceptual model of the flow and distribution of organic carbon in caves. Journal of Cave and Karst Studies 69: 279–284.
- Souza-Silva M, Bernardi LFO, Martins RP, Ferreira RL (2012) Transport and consumption of organic detritus in a Neotropical limestone cave. Acta Carstologica 41: 139–150. https://doi.org/10.3986/ac.v41i1.54
- Thaler K, Knoflach B (2002) Neue Opilioacarus-funde (Acari, Notostigmata) in Peloponnes und Agais (Gricchcnland). Entomologische Nachrichten und Berichte 46: 271–273.
- van der Hammen L (1966) Studies on Opilioacarida (Arachnida) I. Description of Opilioacarus texanus (Chamberlin & Mulaik) and revised classification of the genera. Zoologische Verhandelingen, Leiden, 86: 1–80.
- van der Hammen L (1969) Studies on Opilioacarida (Arachnida) III. Opilioacarus platensis Silvestri, and Adenacarus arabicus (With). Zoologische Mededelingen 44: 113–131.
- Vázquez MM, Klompen H (2002) The family Opilioacaridae (Acari: Parasitiformes) in North and Central America, with description of four new species. Acarologia 42: 299–322.
- Vázquez MM, Klompen H (2009) New species of new world Opilioacaridae (Acari: Parasitiformes) with the description of a new genus from the Caribbean Sternitogenital region. Zootaxa 2061: 23–44. https://doi.org/10.11646/zootaxa.2061.1.2
- Vázquez MM, Klompen H (2010) The genus Salfacarus (Acari: Opilioacarida) in Madagascar. Zootaxa 2482: 1–21. https://doi.org/10.11646/zootaxa.2482.1.1
- Vázquez MM, Klompen H (2015) The family Opilioacaridae (Parasitiformes: Opilioacarida) in Mexico, description of two new species and notes on biology and geographical distribution. Zootaxa 3957: 535–552. https://doi.org/10.11646/zootaxa.3957.5.3
- Vázquez MM, Palacios-Vargas JG (1989) Algumas observaciones sobre el comportamento de los acaros Opilioacaridos (Acari: Notostigmata). Revista Nicaraguense de Entomologia 6: 1–6.
- Vázquez MM, Araújo MS, Feres RJF (2014) A new genus and two new species of Opilioacaridae (Acari: Parasitiformes) from Amazonia, Brazil with a key to world genera. Zootaxa 3814: 151–176. https://doi.org/10.11646/zootaxa.3814.2.1
- Vázquez MM, May D, Alamilla E, Klompen H (2018) A new species of Opilioacaridae (Parasitiformes: Opilioacarida) from Belize with some observations on life history and behavior. Systematic and Applied Acarology 23: 132–144. https://doi.org/10.11158/saa.23.1.11
- Vázquez MM, Bernardi LFO, Klompen H (2020) The family Opilioacaridae (Acari: Parasitiformes) in Argentina, with description of two new species. Acarologia 60: 505–519.
- Walter DE, Krantz GW (2009) Collection, rearing, and preparing specimens. In: Krantz GW, Walter DE (Eds) , Manual of Acarology. Texas Tech University Press, Lubbock, 83–96.
- Walter DE, Proctor HC (1998) Feeding behaviour and phylogeny: observations on early derivative Acari. Experimental and Applied Acarology 22: 39–50. https://doi.org/10.1023/A:1006033407957
- Wynne JJ, Howarth FG, Sommer S, Dickson BG (2019) Fifty years of cave arthropod sampling: techniques and best practices. International Journal of Speleology 48: 33–48. https://doi.org/10.5038/1827-806X.48.1.2231